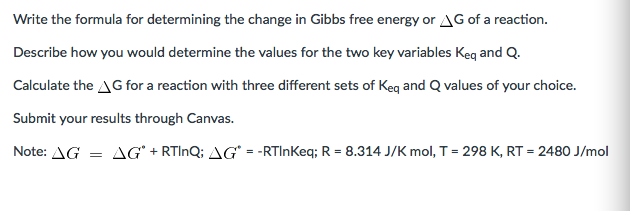
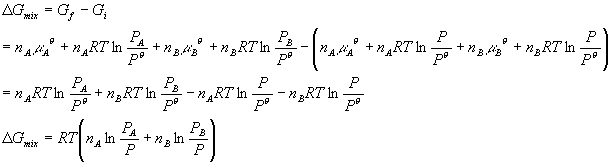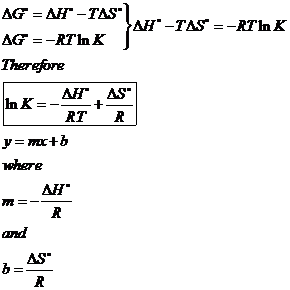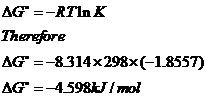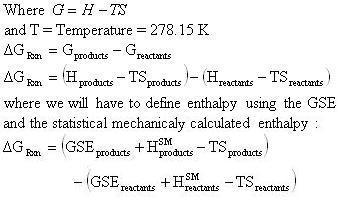Delta G Formula
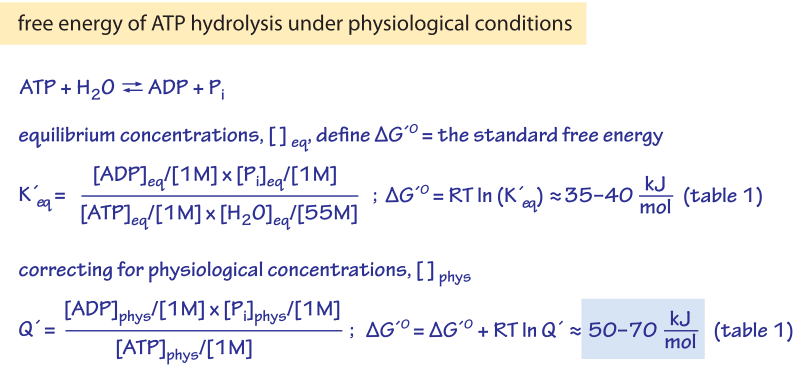
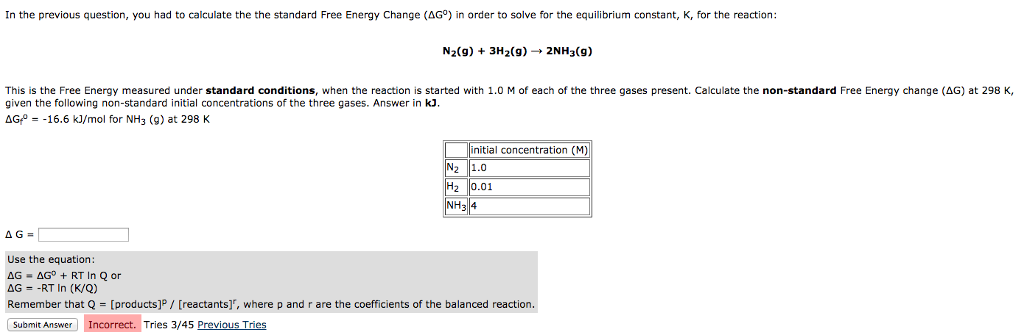
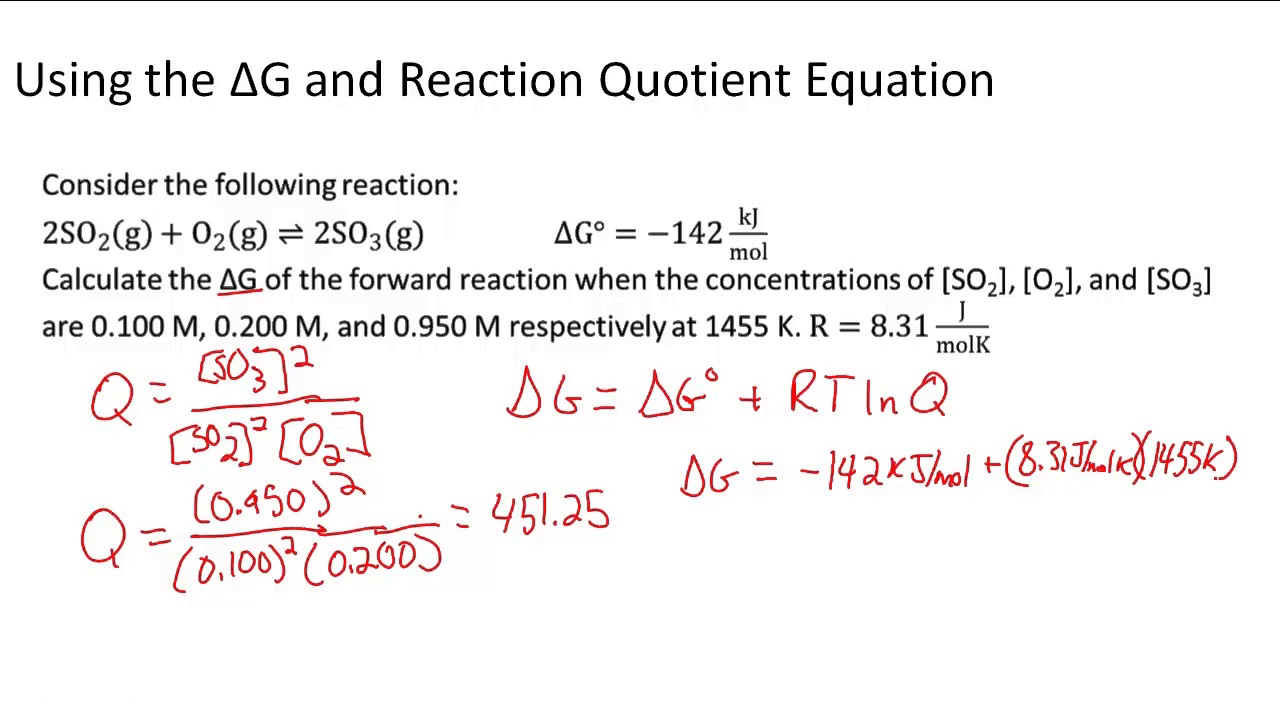
This is the free energy change for a reaction that is not at the standard state.

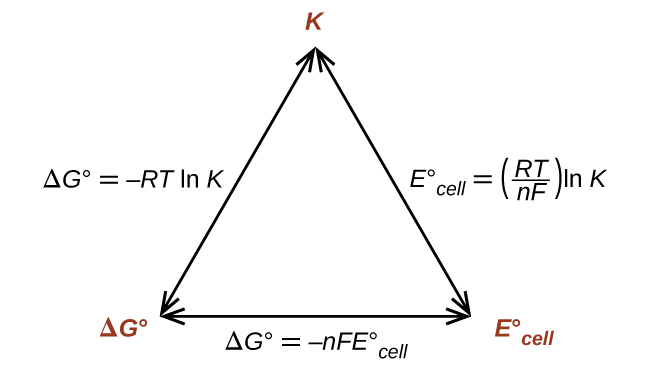
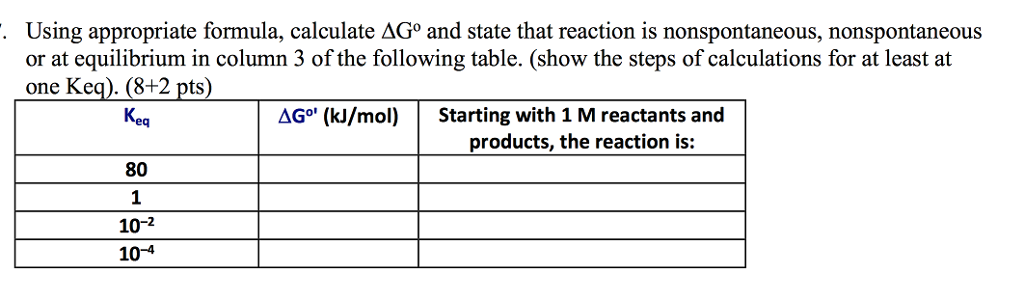
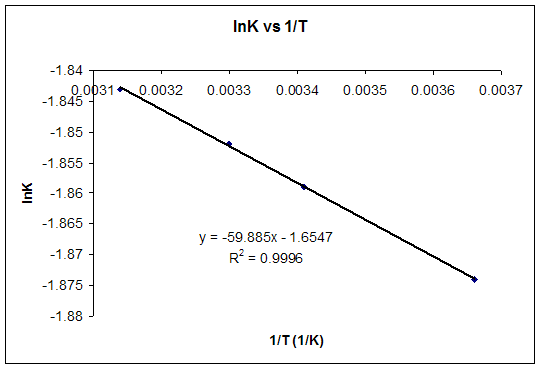
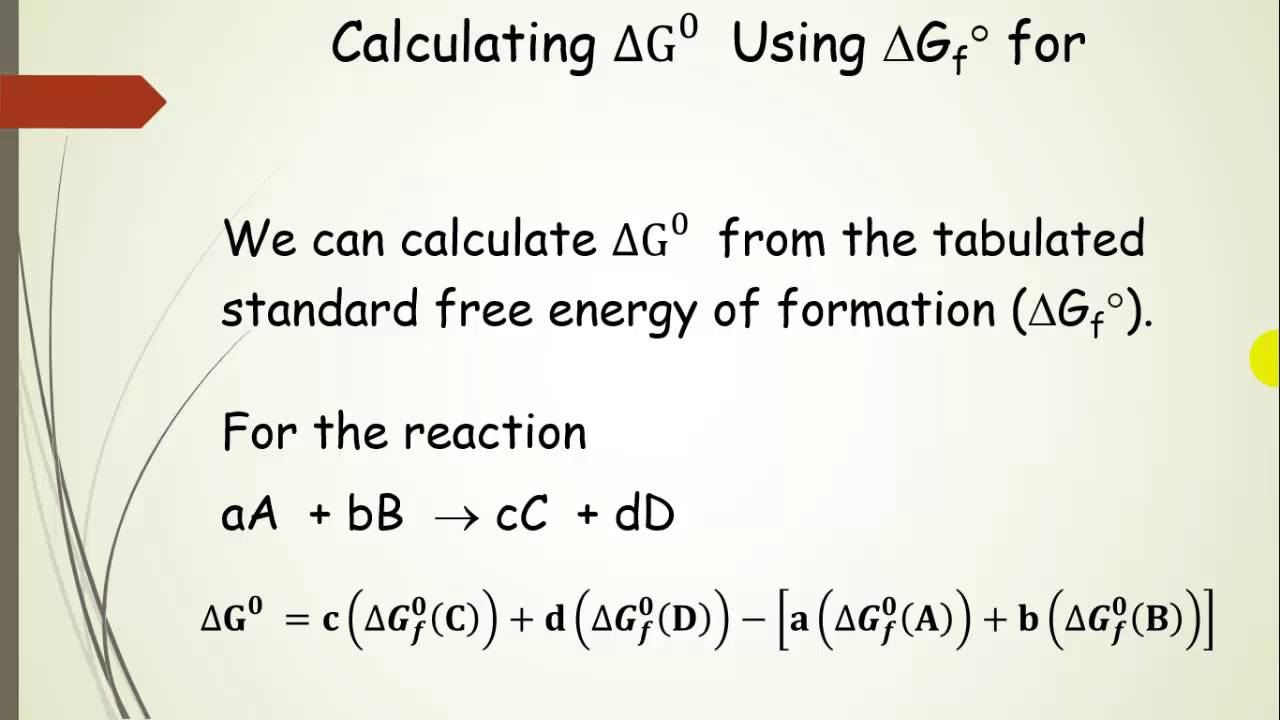
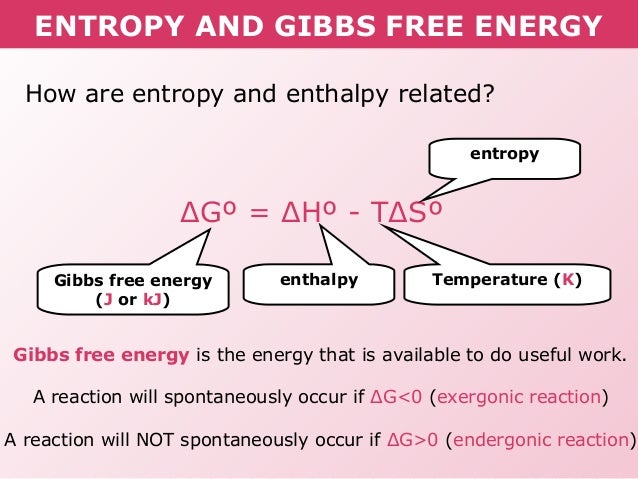
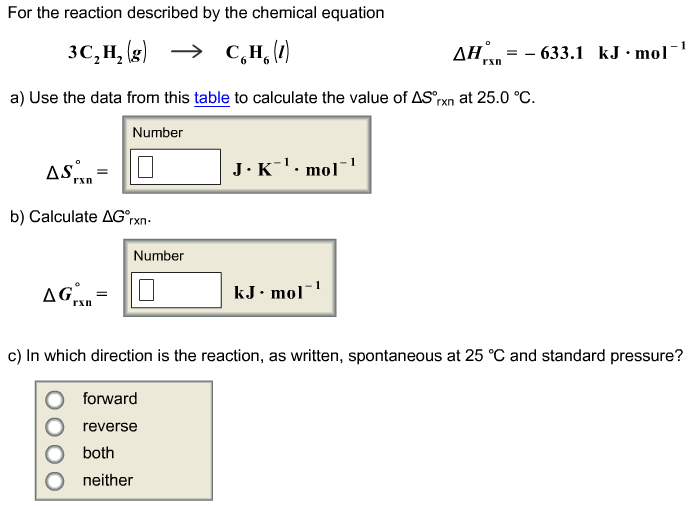
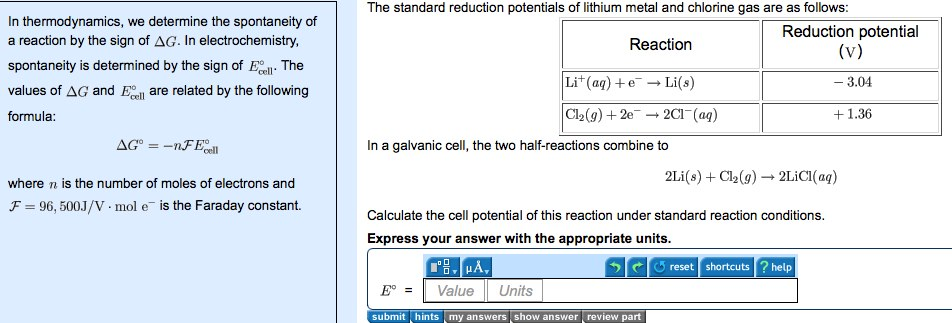
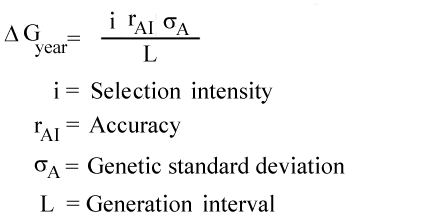
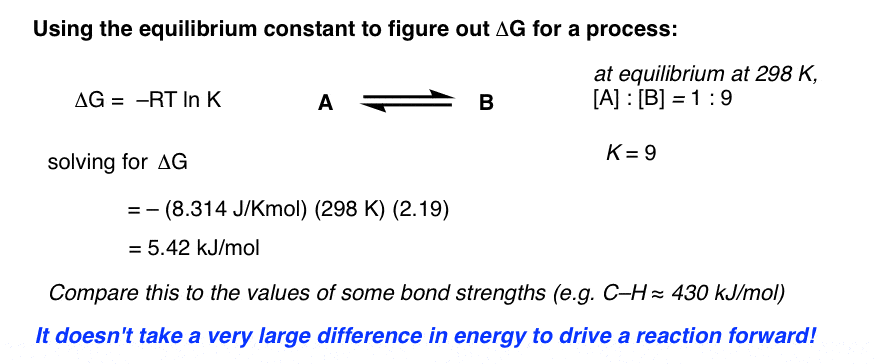
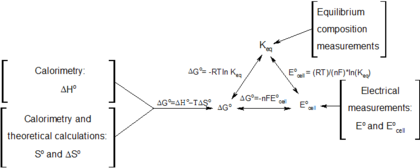
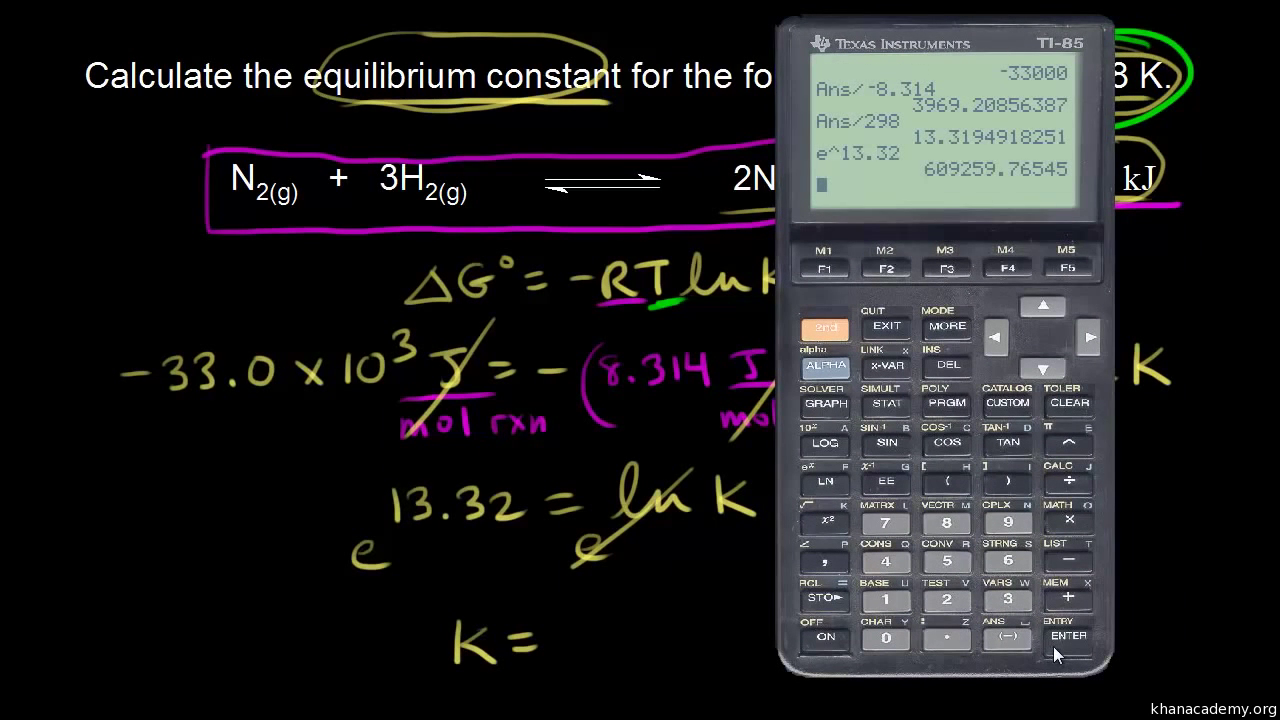
Delta g formula. R 8314 j mol 1 k 1 or 0008314 kj mol 1 k 1. If we know the standard state free energy change g o for a chemical process at some temperature t we can calculate the equilibrium constant for the process at that temperature using the relationship between g o and k. The entropy term is therefore subtracted from the enthalpy term when calculating g o for a reaction.
As such i think that knowledge of it and the consequences associated with it are. However again because all of these are linked to chemistry and because chemistry likes to measure everything per mole all of the variables above but temperature may also have attached to them per mole mol. Delta g delta h t delta s chad explains the relationship between gibbs free energy enthalpy and entropy and when a reaction will be spontaneous.
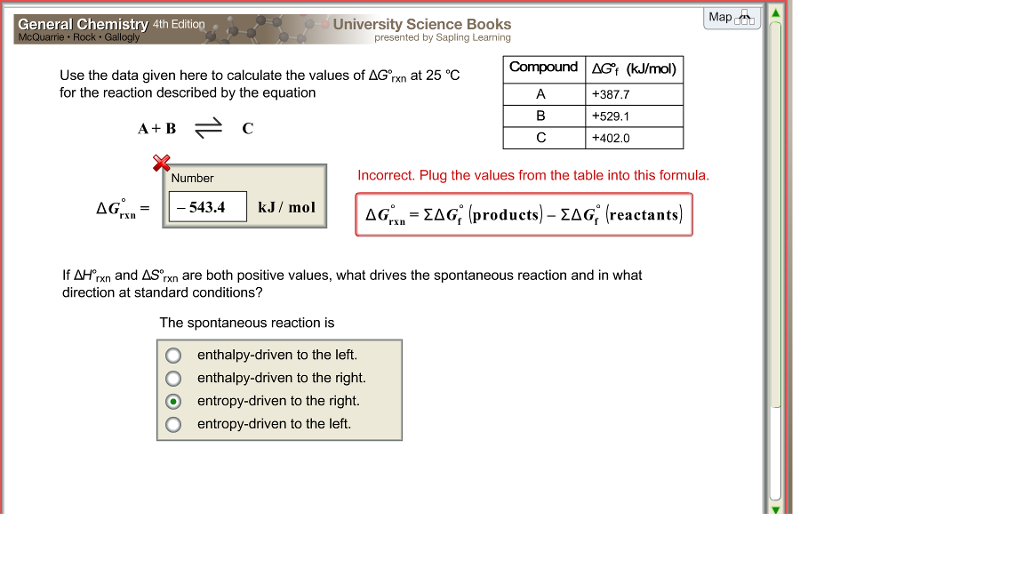
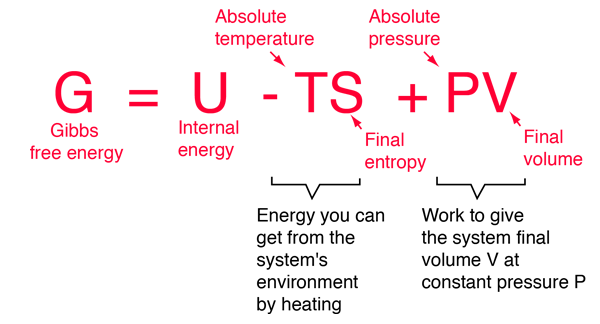
Rearrangement gives in this equation. Since this post was originally written in january 2012 the ap exam has changed. D g o a delta g with a superscript o is the free energy change for a reaction with everything in the standard states gases at 1 bar and solutions at 1 m concentration and at a specific temperature usually 250c d g just delta g.
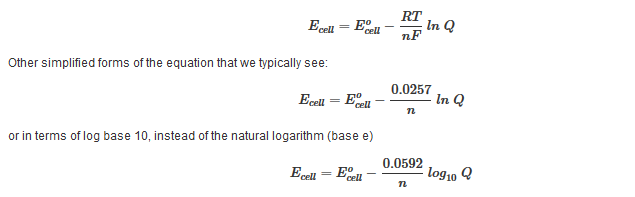
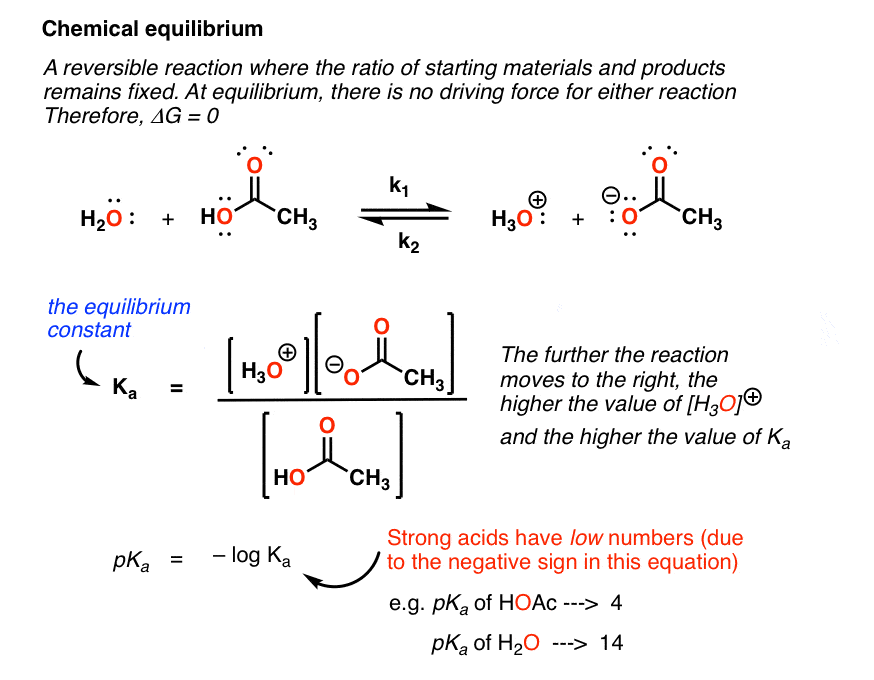
A quantitative measure of the favorability of a given reaction at constant temperature and pressure is the change dg sometimes written delta g. T is the temperature on the kelvin. D s is in the units of joules per kelvin j k.
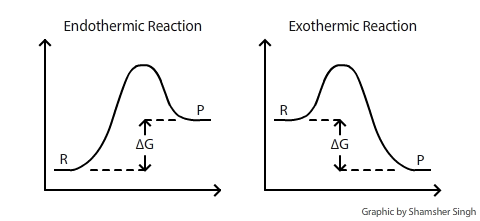
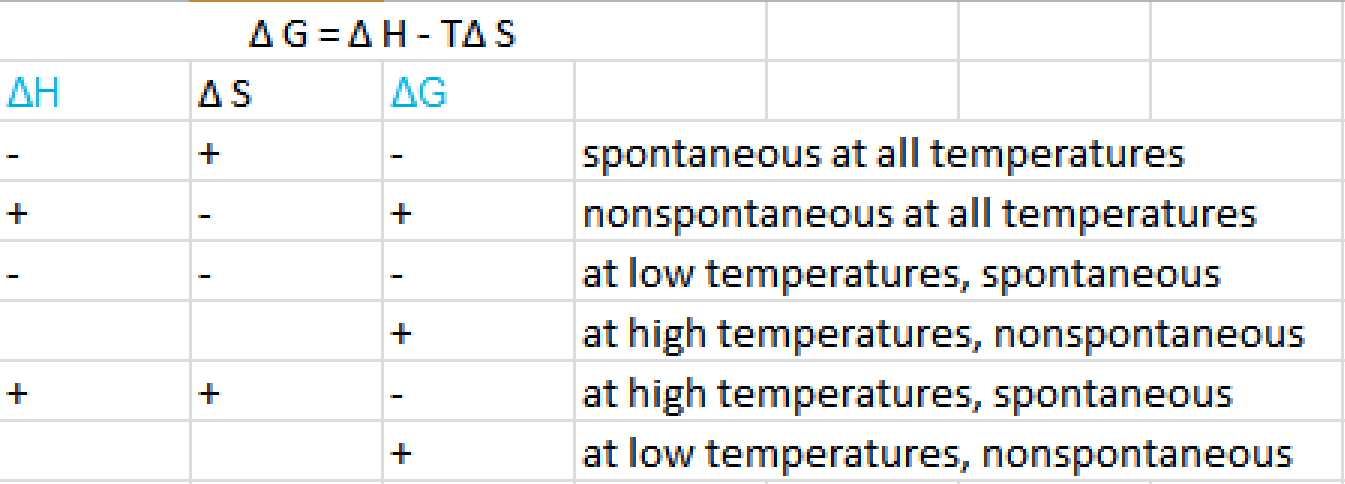
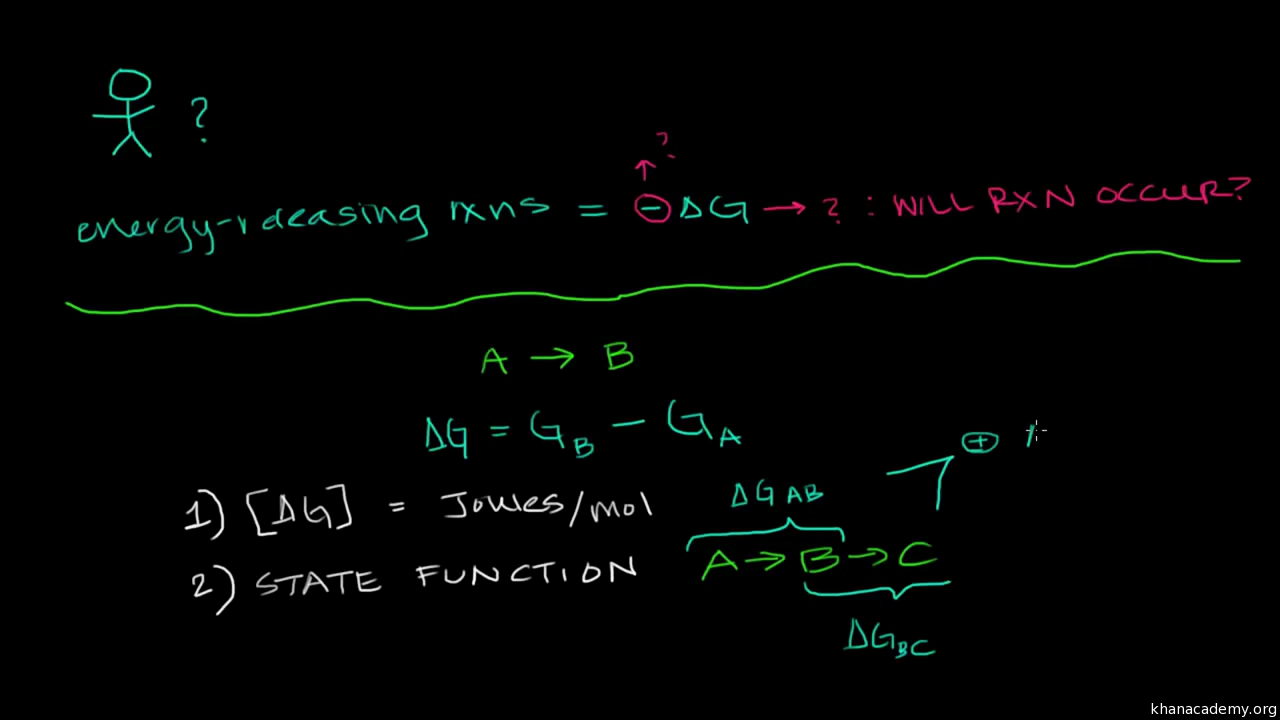
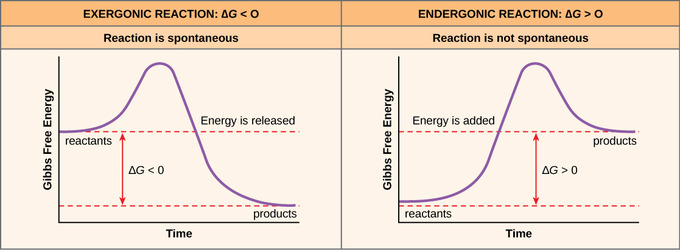
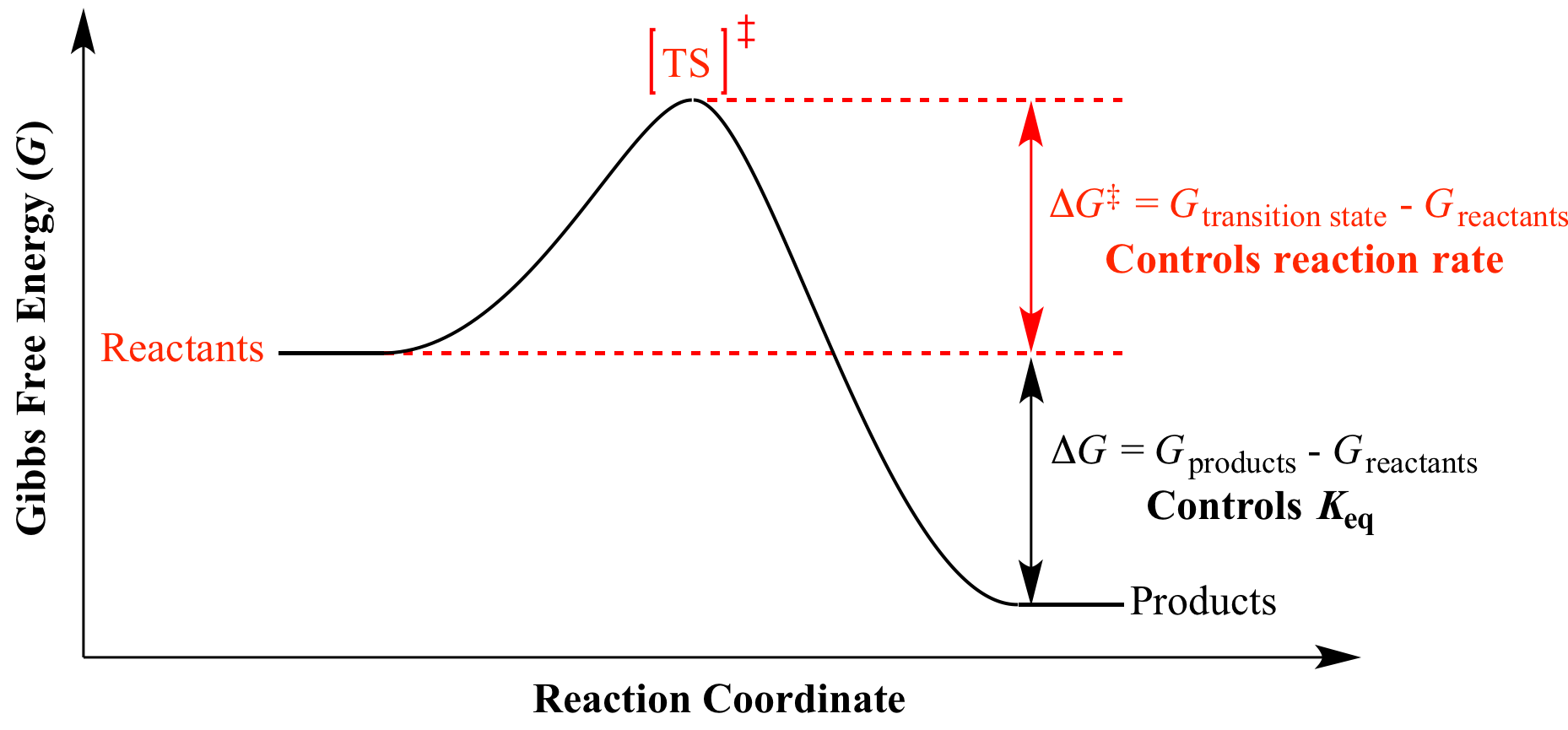
How the second law of thermodynamics helps us determine whether a process will be spontaneous and using changes in gibbs free energy to predict whether a reaction will be spontaneous in the forward or reverse direction or whether it is at equilibrium. According to the second law of thermodynamics for systems reacting at standard conditions for temperature and pressure or any other fixed temperature and pressure there is a general natural tendency to achieve a minimum of the gibbs free energy. We can therefore conclude that any reaction for which.
Consider the two equations that deal with delta g g. G o is therefore negative for any reaction that is favored by both the enthalpy and entropy terms. Because of the way the free energy of the system is defined g o is negative for any reaction for which h o is negative and s o is positive.
D g is in the units joules j. Delta can be positive or negative being between 0 and 1 for a call. T is in the units of kelvin k.

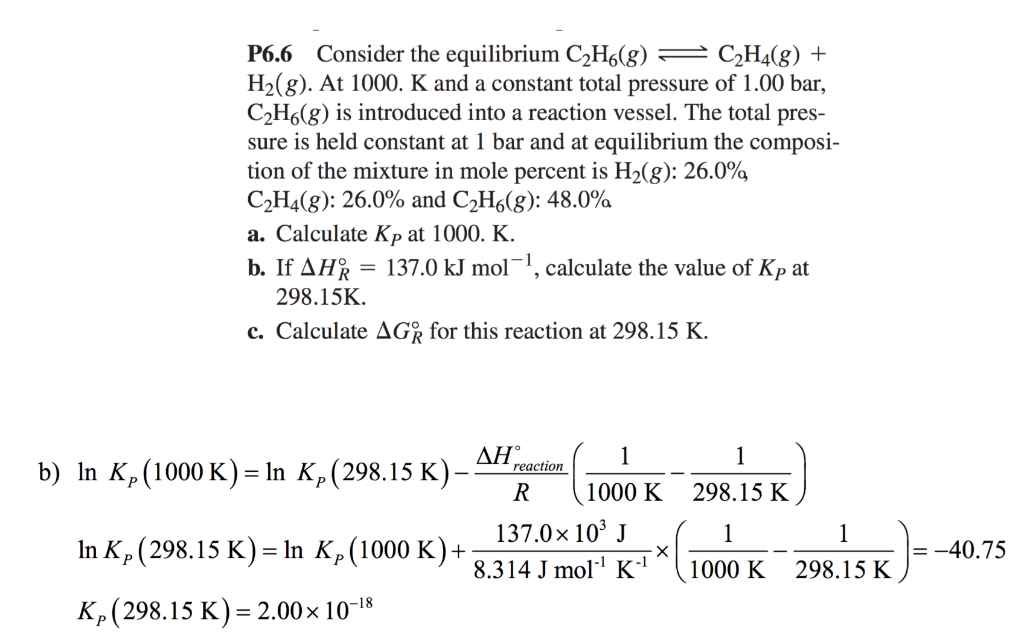
The Relationship Between Free Energy And The Equilibrium Constant Video Lesson Transcript Study Com
study.com
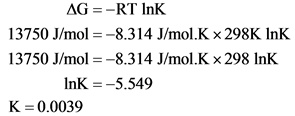
What Is The Value Of K For This Aqueous Reaction At 298 K Delta G 13 75 K Mol Home Work Help Learn Cbse Forum
ask.learncbse.in