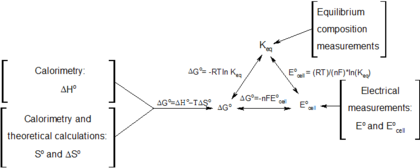
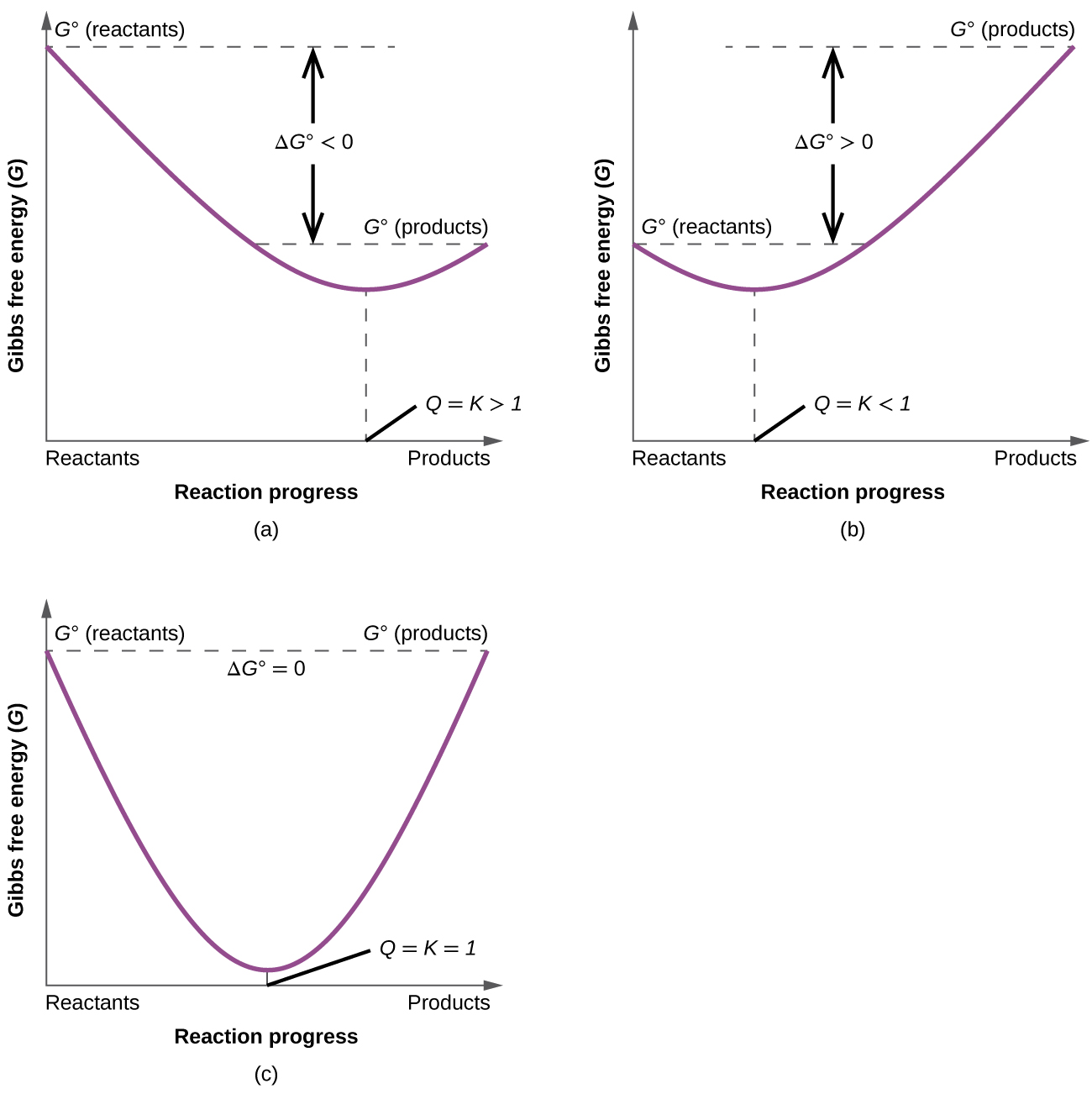
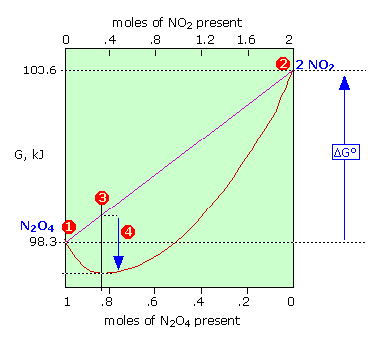
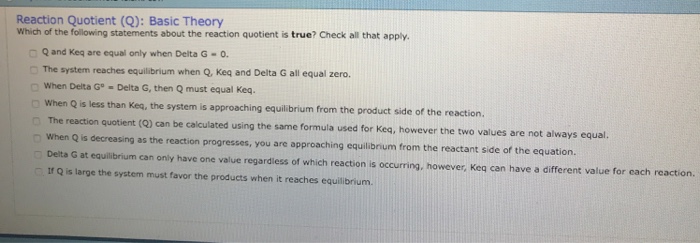
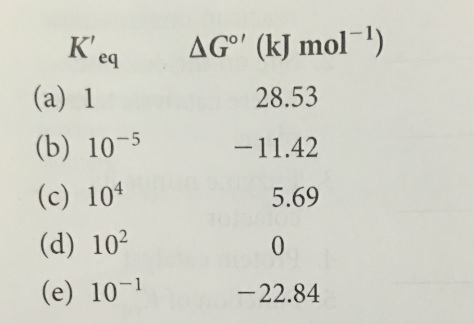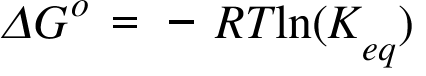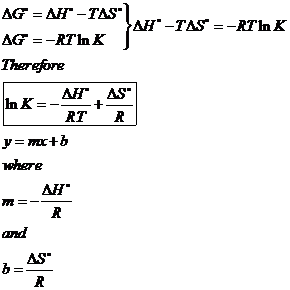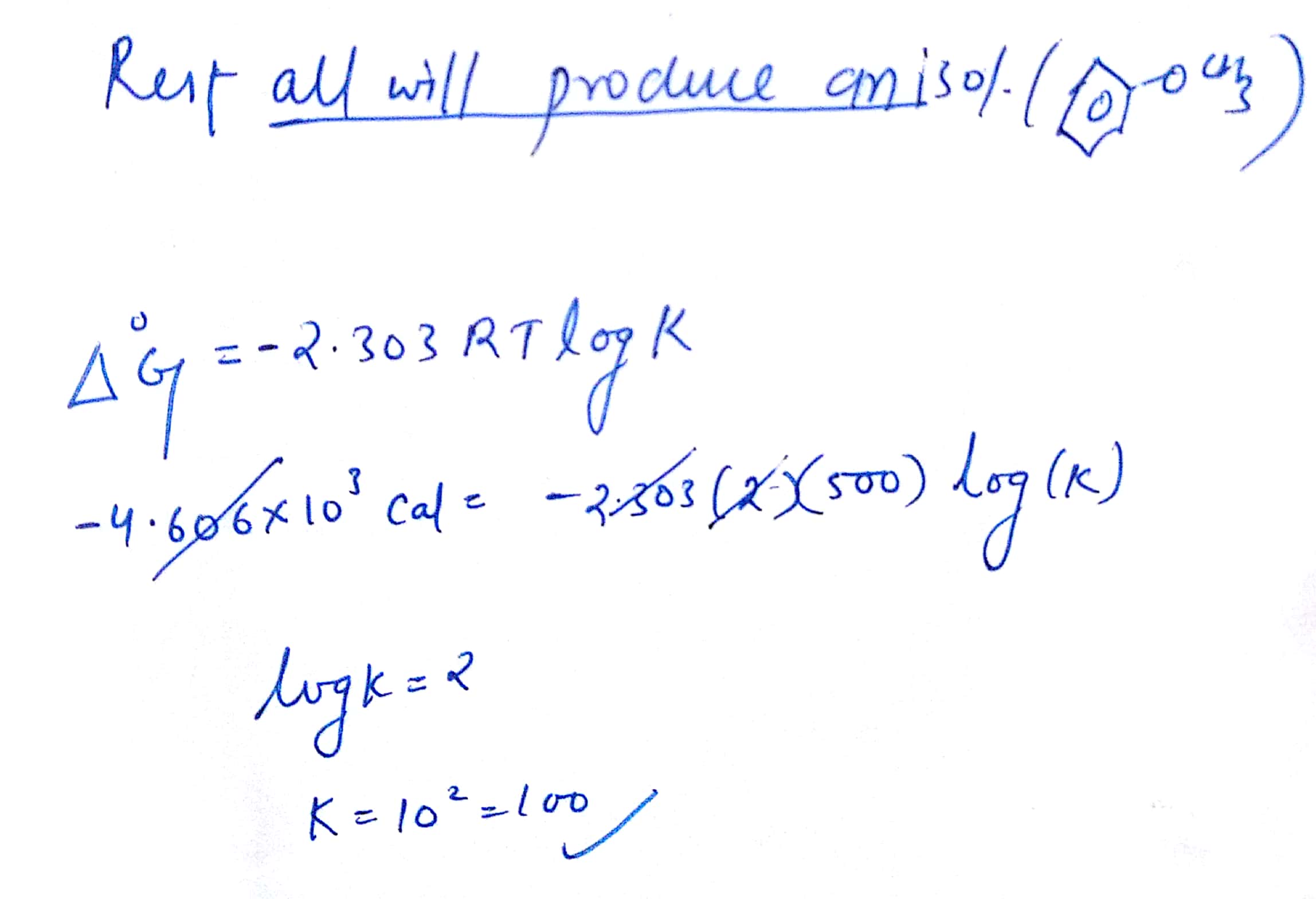Keq Formula Delta G
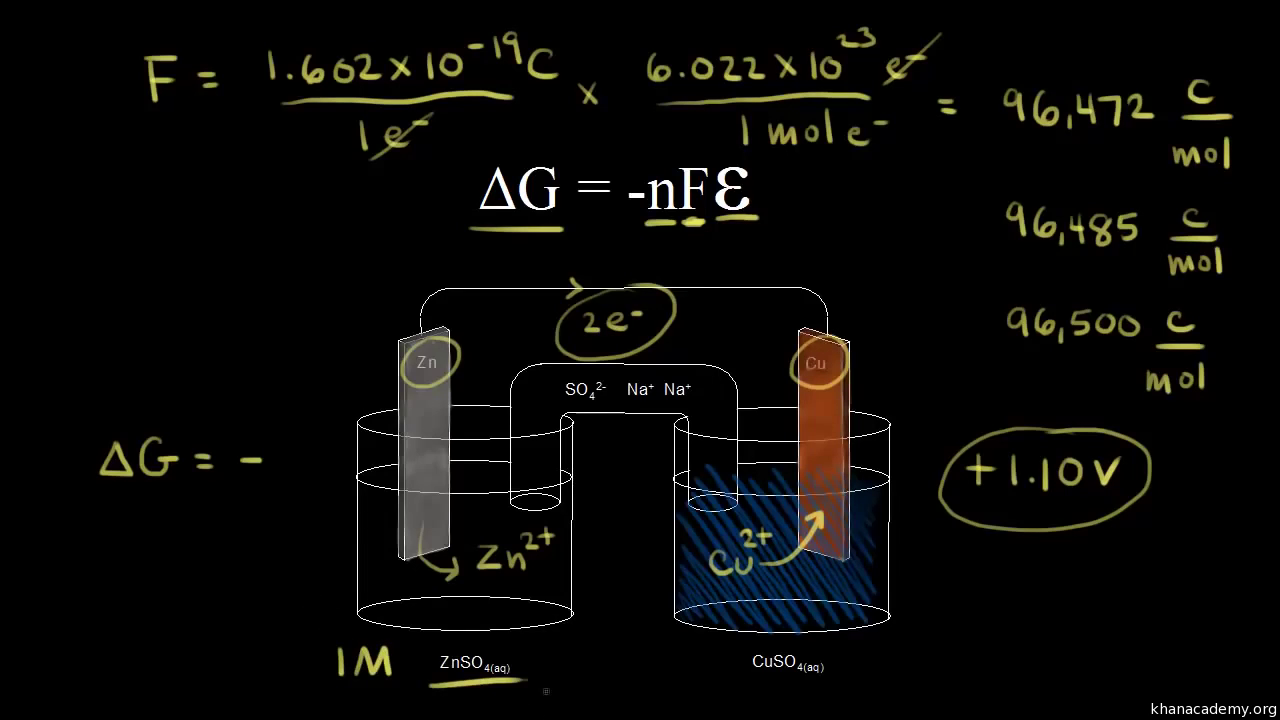
Consider the two equations that deal with delta g g.

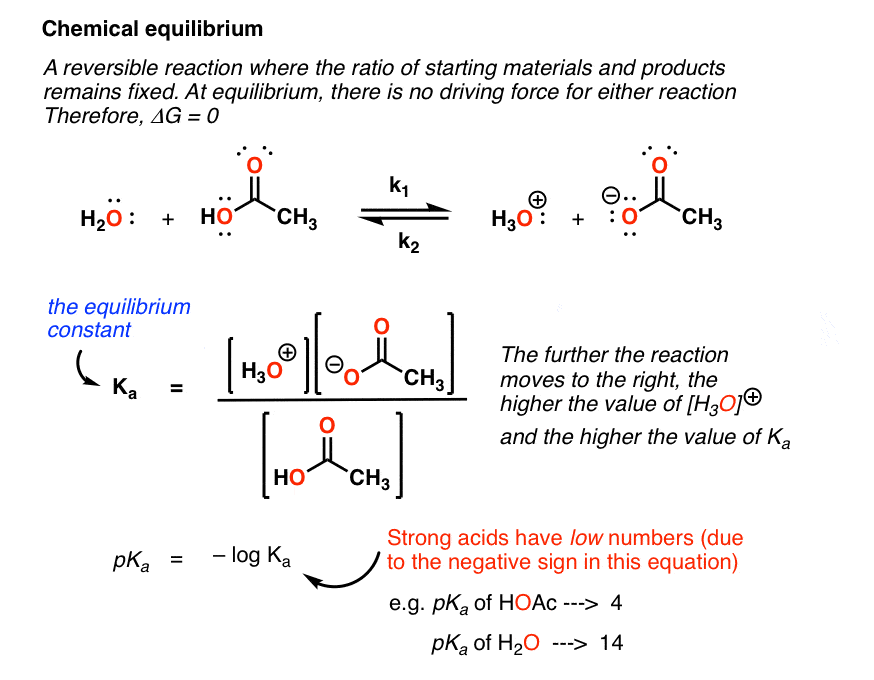
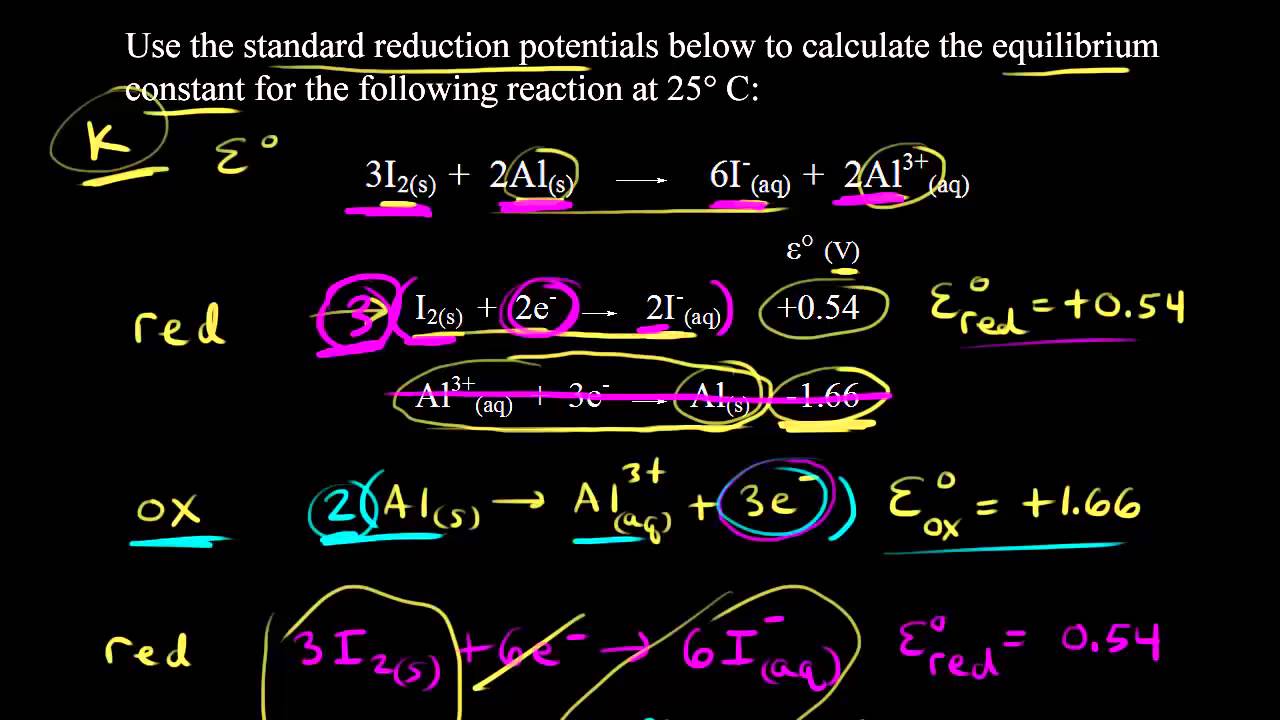
Keq formula delta g. The keq expressions for solids and liquids. One of the changes was to remove equation 2 below from the equations constants sheet. Raise both sides to the exponential e.
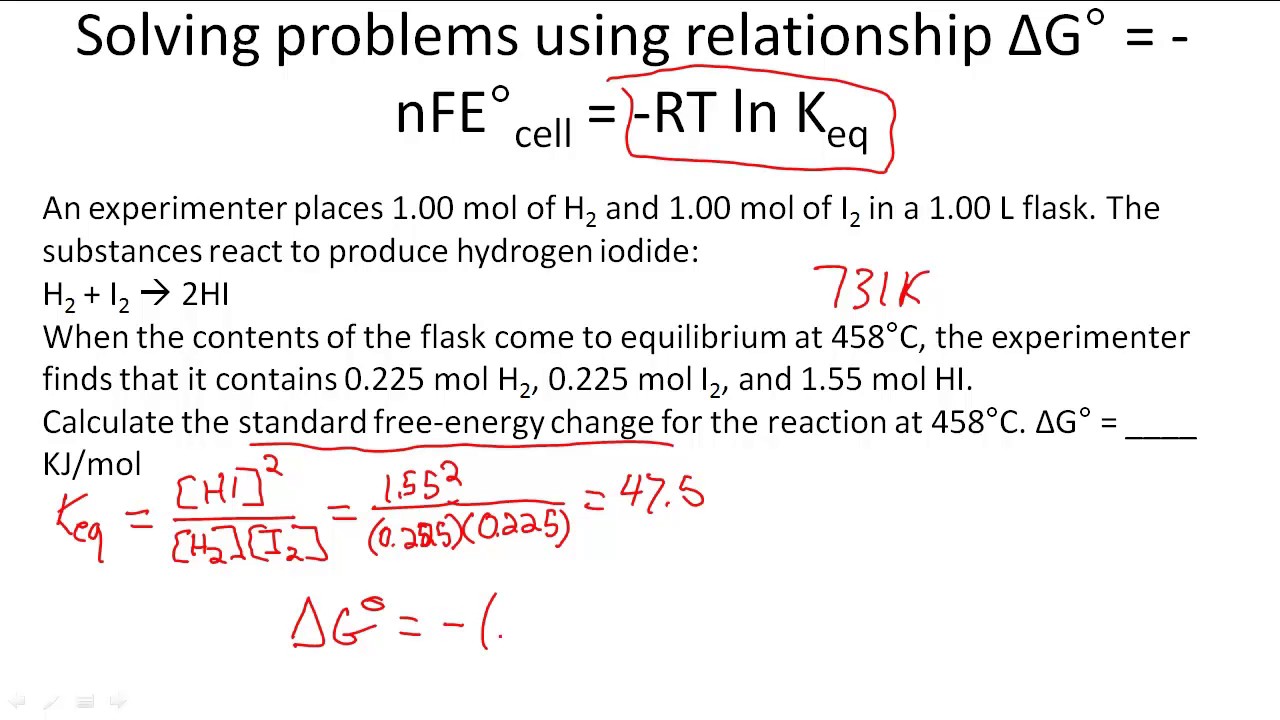
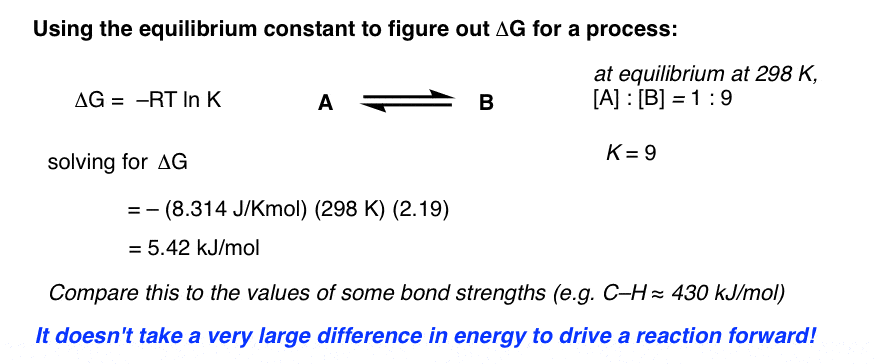
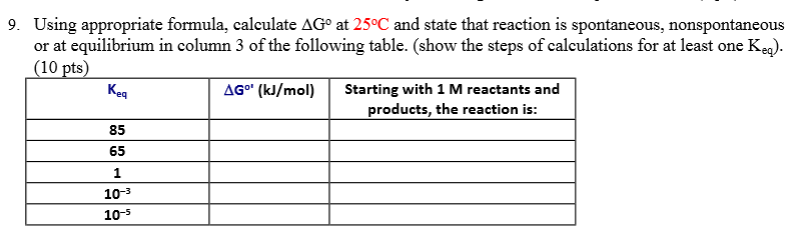
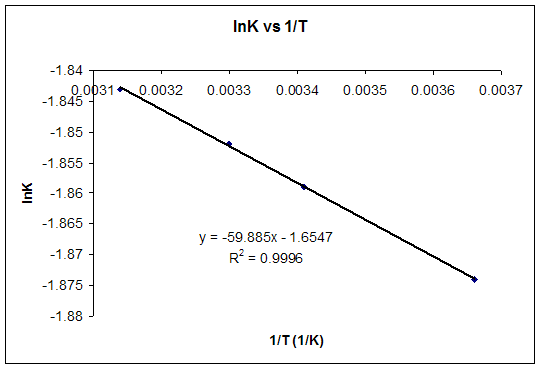
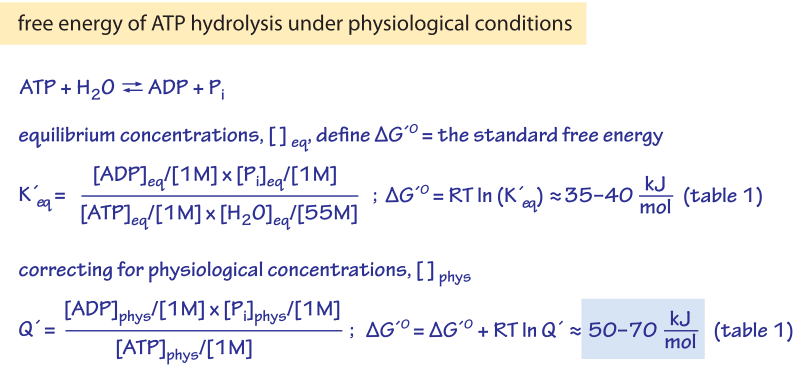
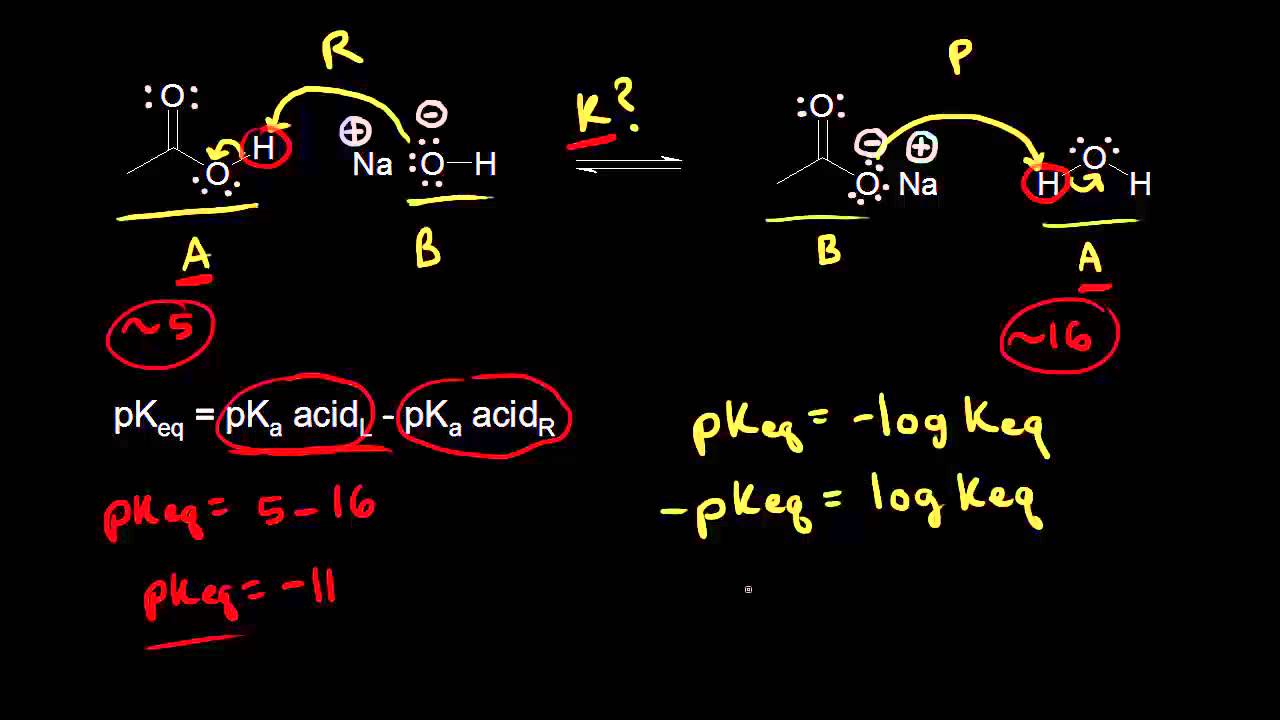
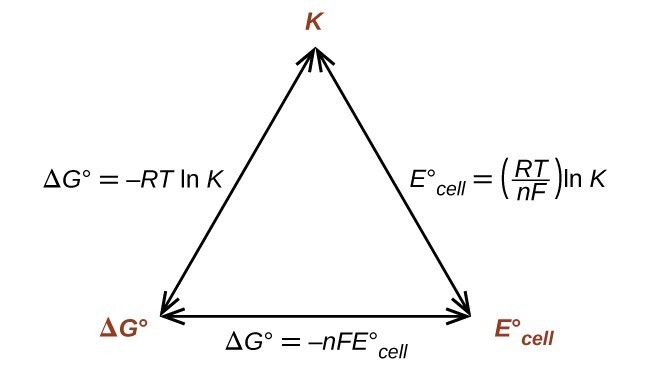
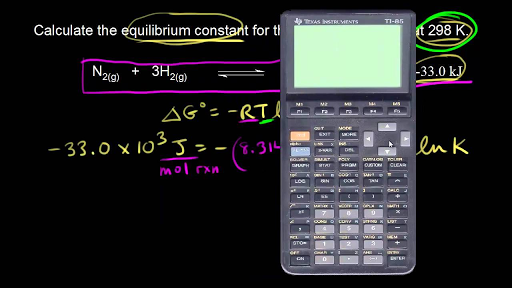
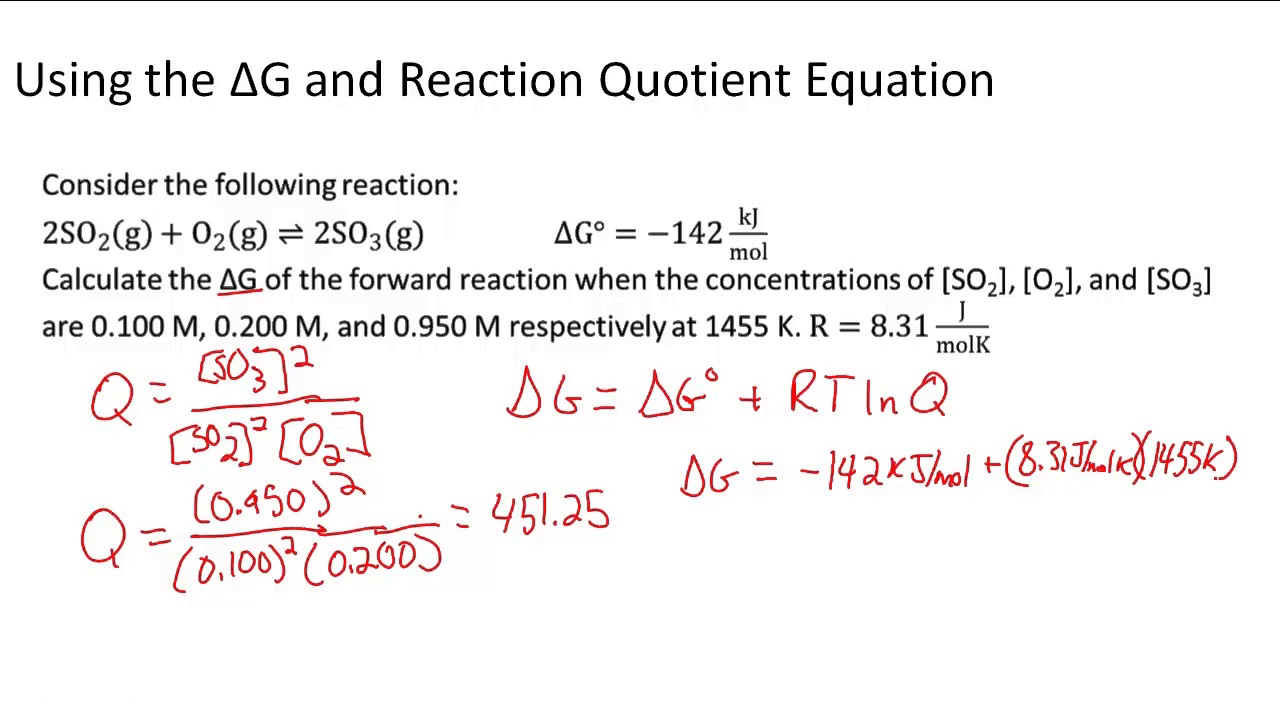
If we know the standard state free energy change g o for a chemical process at some temperature t we can calculate the equilibrium constant for the process at that temperature using the relationship between g o and k. 23027 ln keq. Rearrangement gives in this equation.
As such i think that knowledge of it and the consequences associated with it are. T is the temperature on the kelvin. Add 273 to the temperature to convert to kelvin 25 273 298 k.
Here you denote the molar concentration of a substance by writing its formula is square bracketsthe subscript eq on the equilibrium contant means that it is defined in terms of molar concentrations. E23027 e ln keq. G rtlnkeq multiply delta g by 1000 to convert to jmol 57050 jmol.
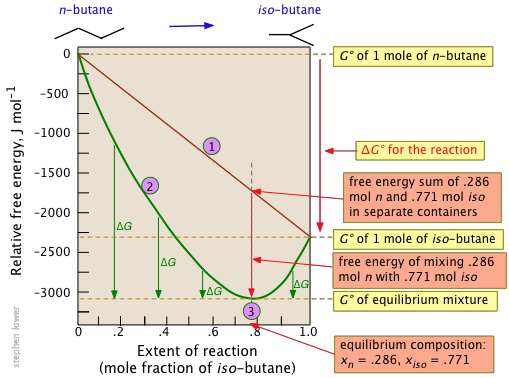
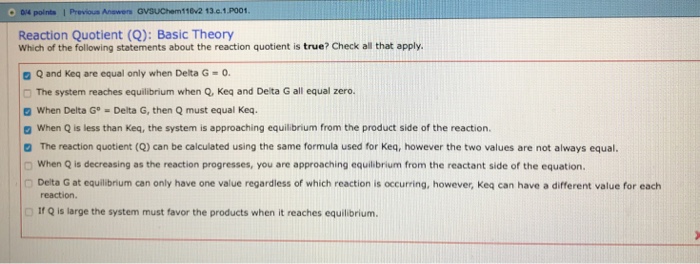
100 e10 final. The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency towards further changefor a given set of reaction conditions the equilibrium constant is independent of the initial analytical concentrations of the. R 8314 j mol 1 k 1 or 0008314 kj mol 1 k 1.