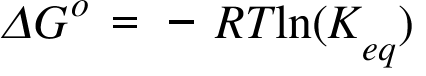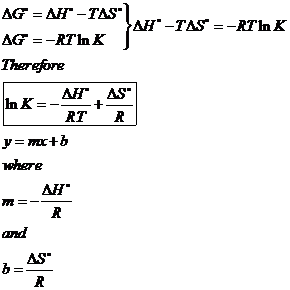Delta G Naught Formula
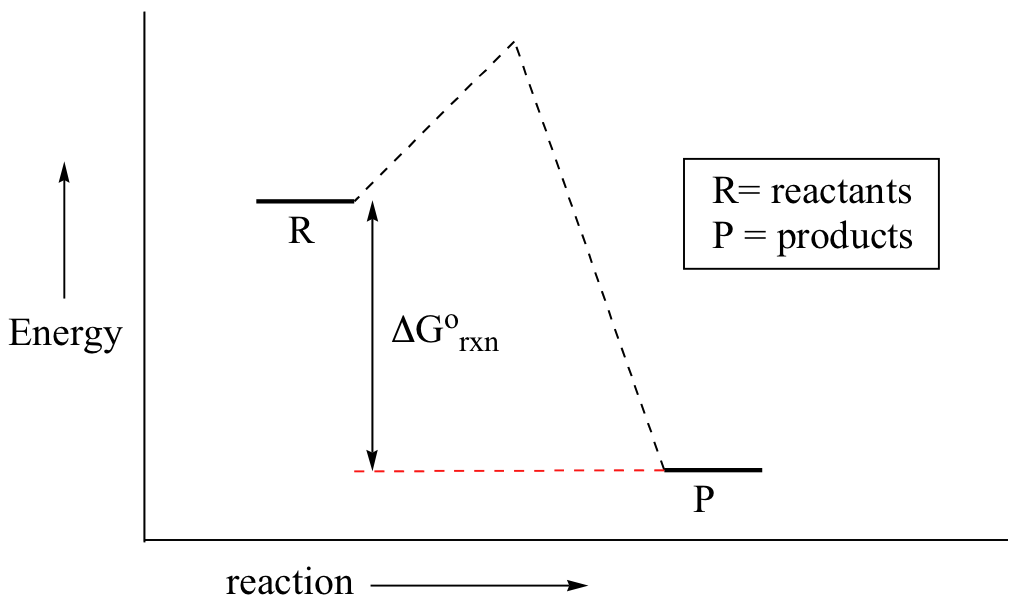
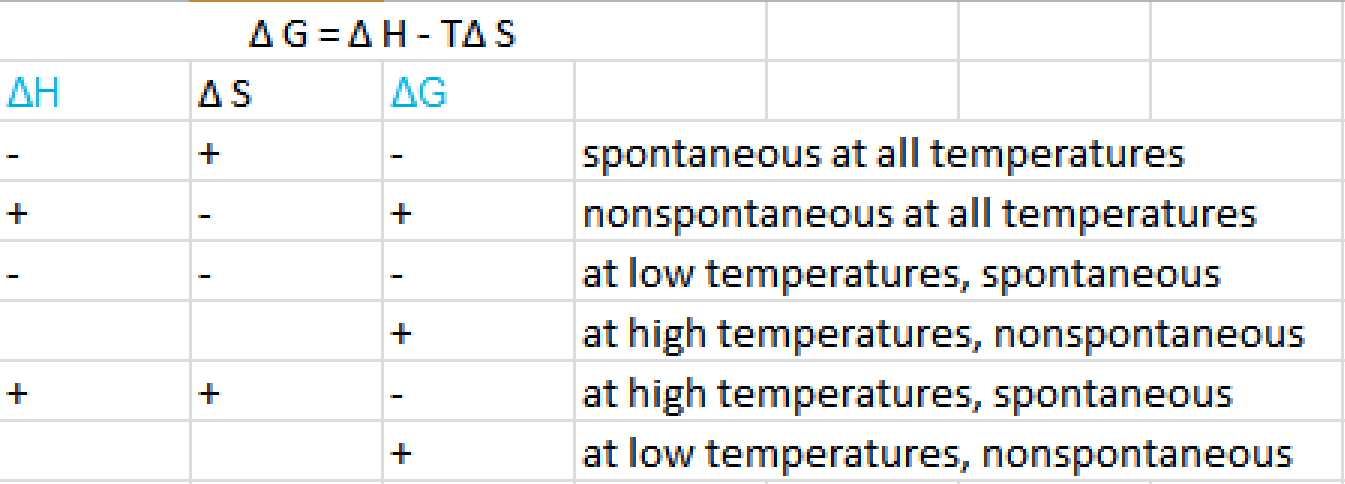
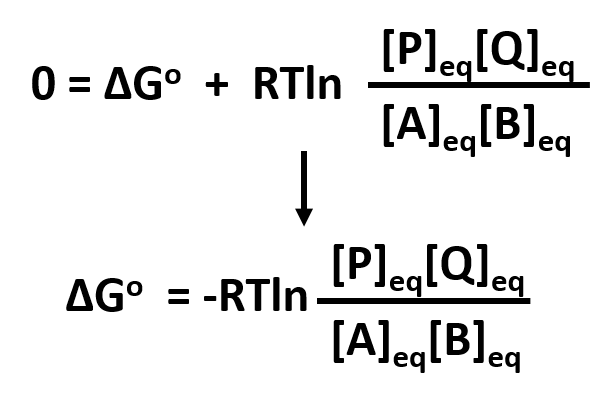
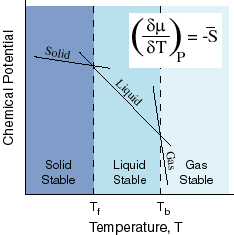
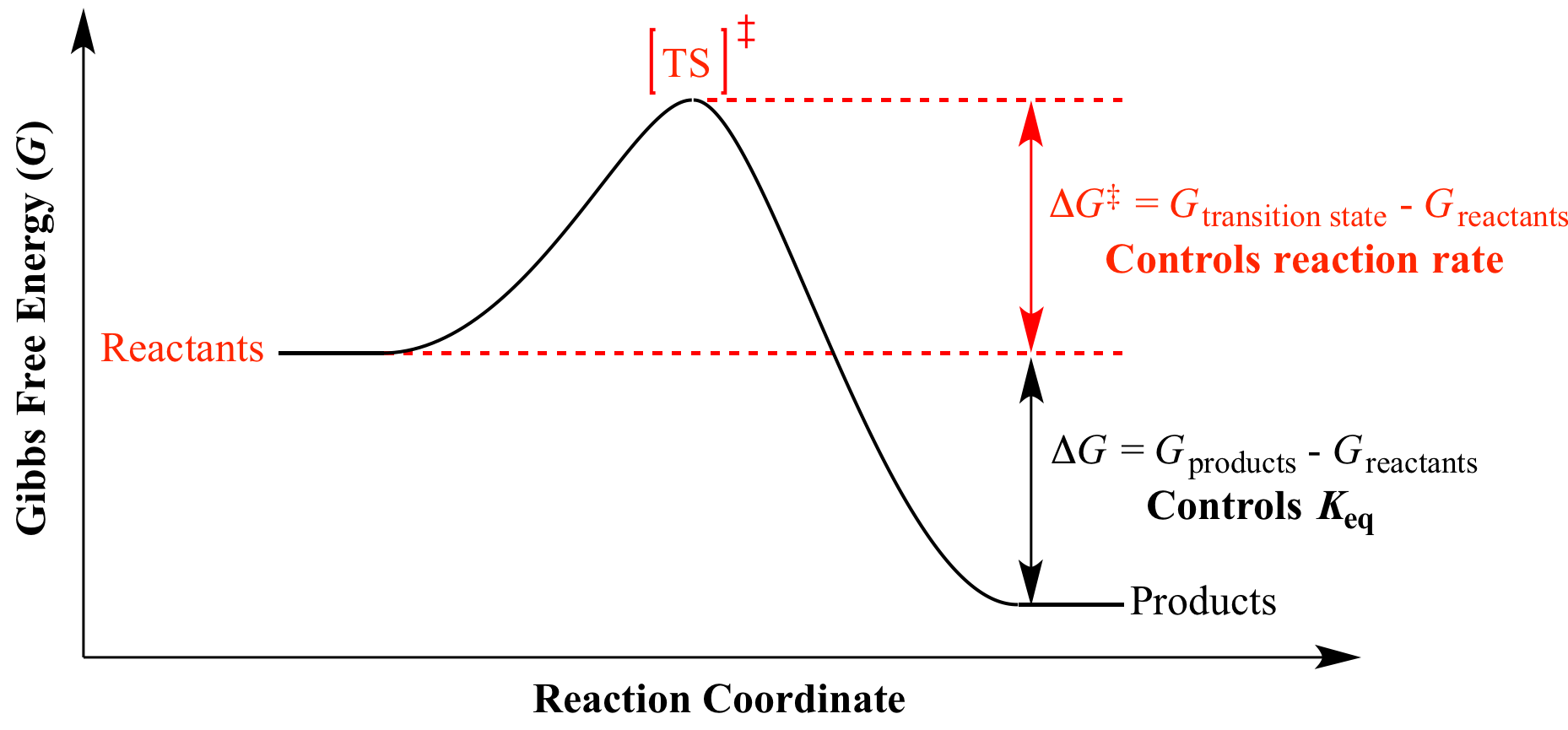
The smaller the value of g o the closer the standard state is to equilibrium.
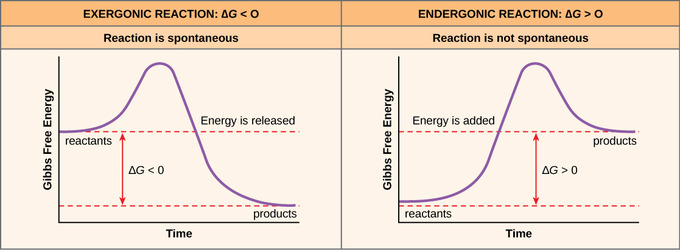
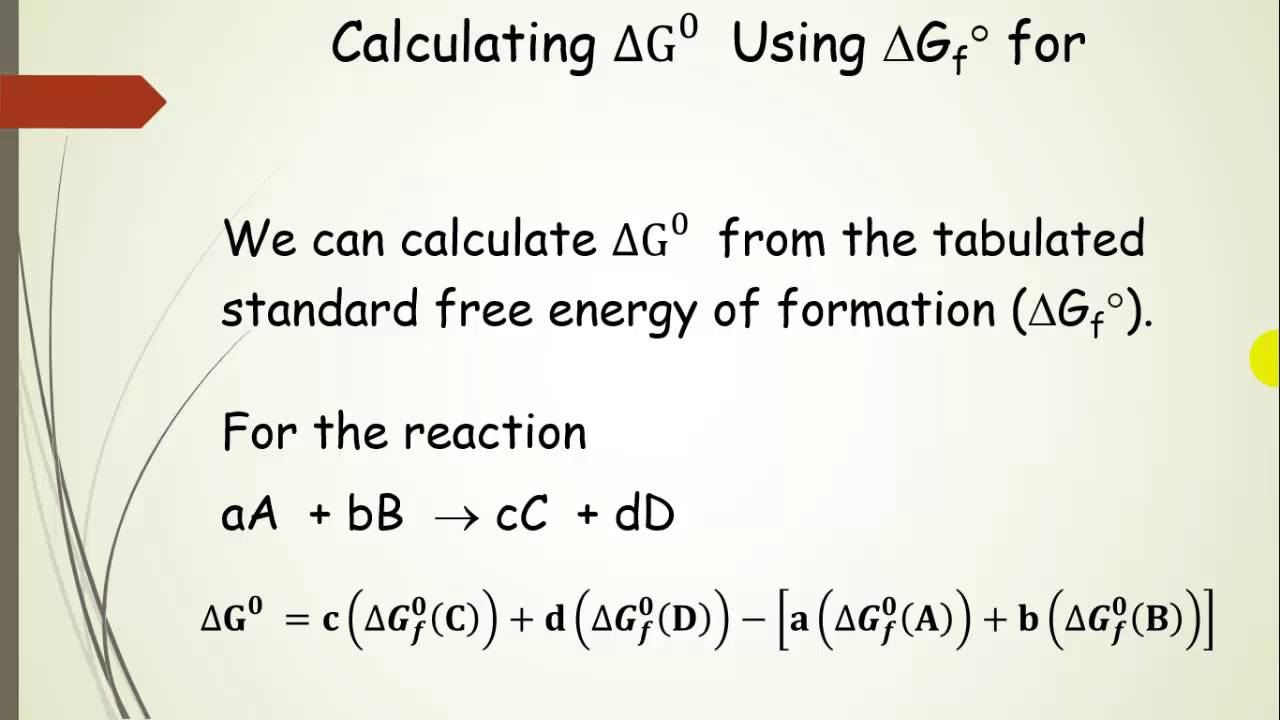
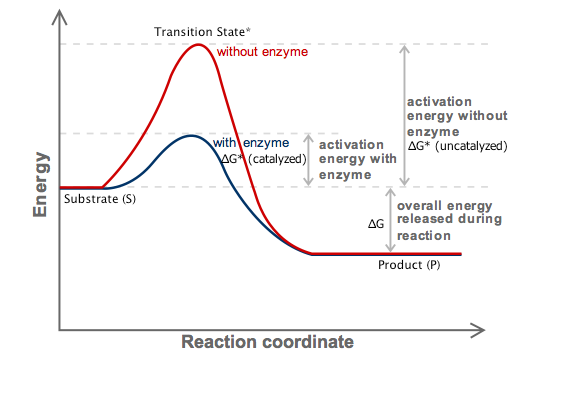
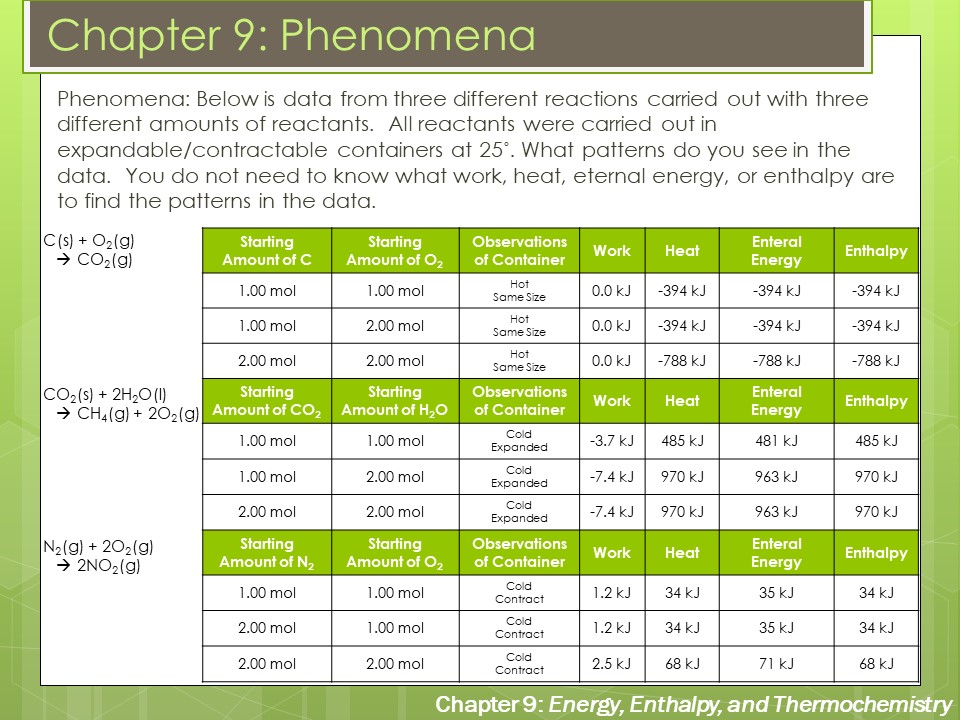
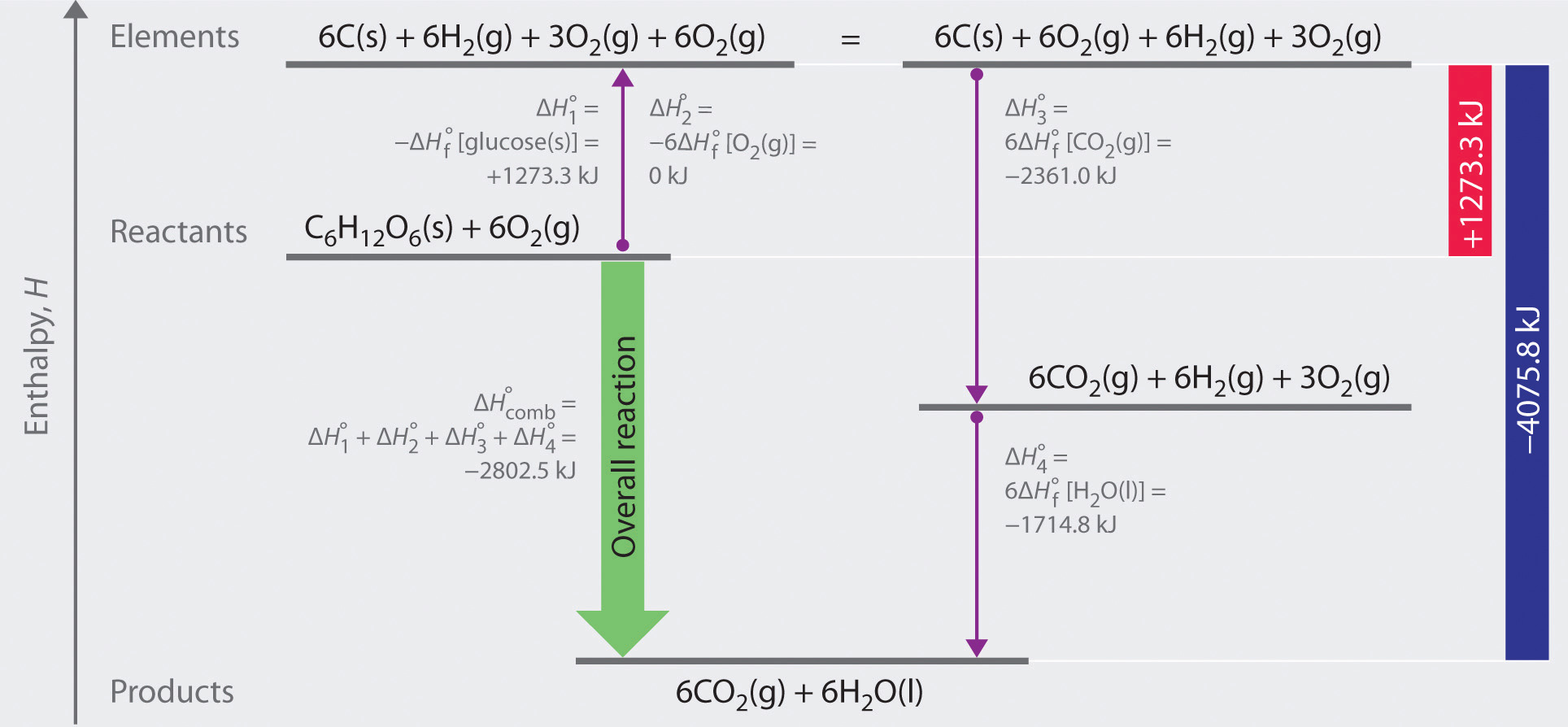
Delta g naught formula. Rearrangement gives in this equation. According to the second law of thermodynamics for systems reacting at standard conditions for temperature and pressure or any other fixed temperature and pressure there is a general natural tendency to achieve a minimum of the gibbs free energy. Delta g delta h t delta s chad explains the relationship between gibbs free energy enthalpy and entropy and when a reaction will be spontaneous.
I just had a question that has been really bothering me about the difference between delta g naught and delta d. R 8314 j mol 1 k 1 or 0008314 kj mol 1 k 1. So delta g naught is constant for a given reaction.
A quantitative measure of the favorability of a given reaction at constant temperature and pressure is the change dg sometimes written delta g. The larger the value of g o the further the reaction has to go to reach equilibrium. But delta g naught is the delta g at standard condition.
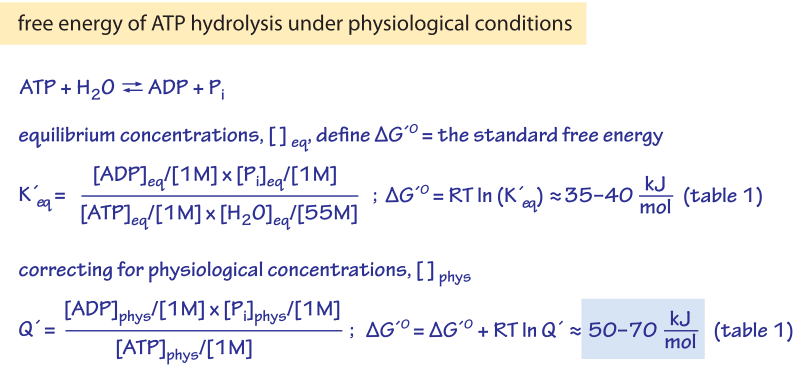
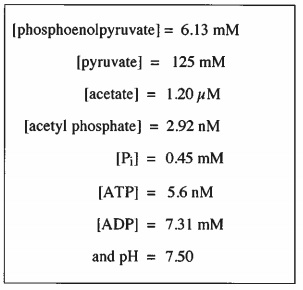
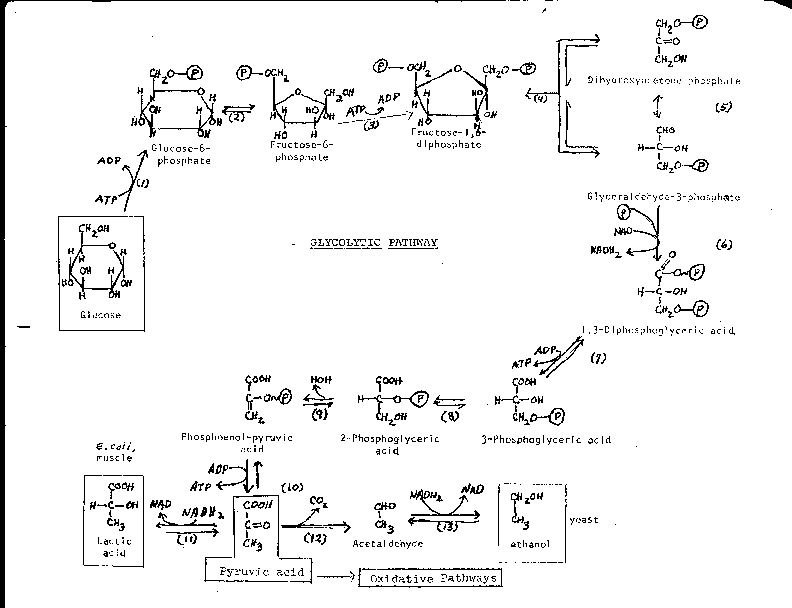
Delta g naught prime means that the ph is 7 physiologic conditions everything else is the same. The concentration of h now isnt 1 molar because 1 molar concentration would be an extremely low ph 0. Delta g naught means that the reaction is under standard conditions 25 celsius 1 m concentraion of all reactants and 1 atm pressure.
It is related to k at the equilibrium temp since then delta g is 0. For example under standard conditions the reaction of cos with ni 2 aq to form nis and co 2 aq occurs spontaneously but if we reduce the concentration of ni 2 by a factor of 100 so that ni 2 is 001 m then the reverse reaction occurs spontaneously instead. Values of g o and k for common reactions at 25 o c.
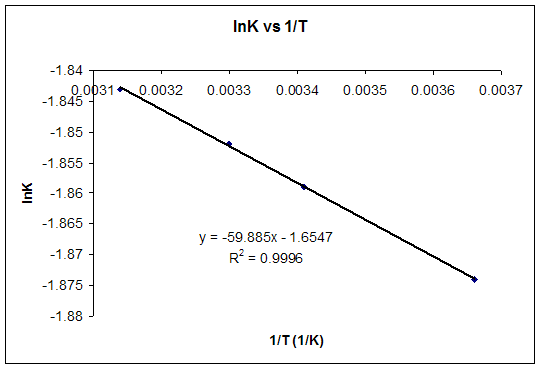
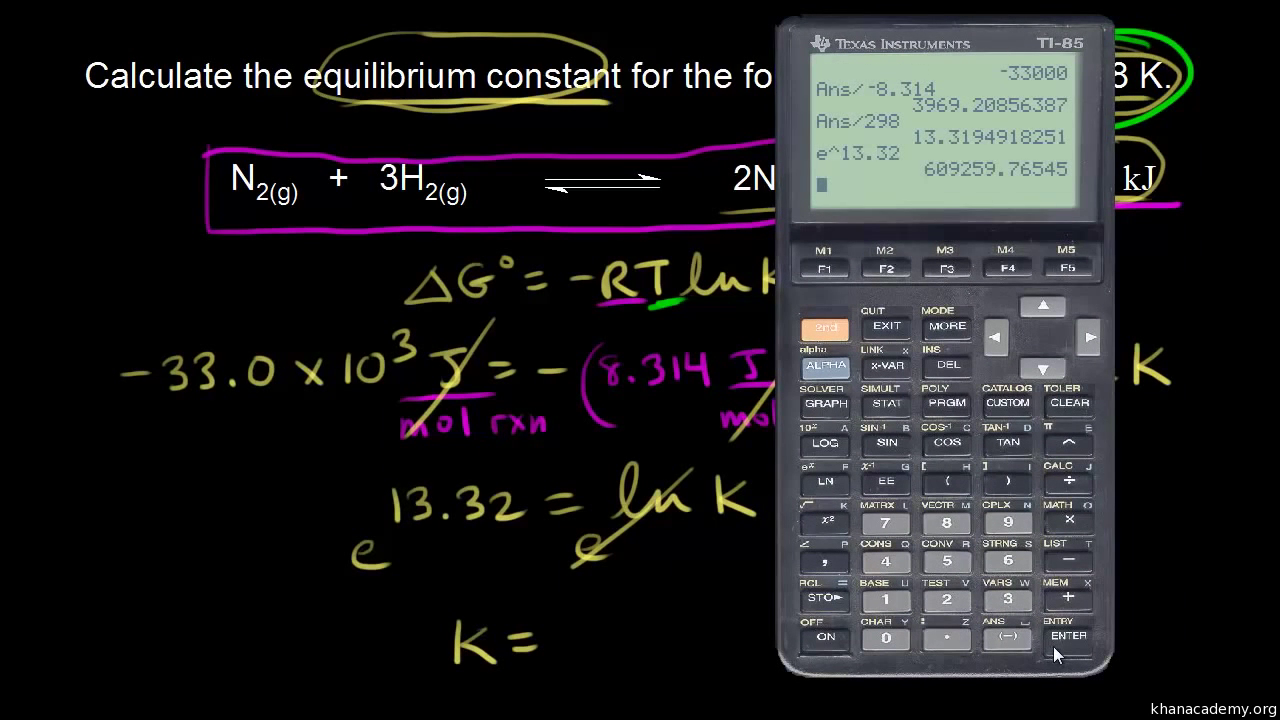
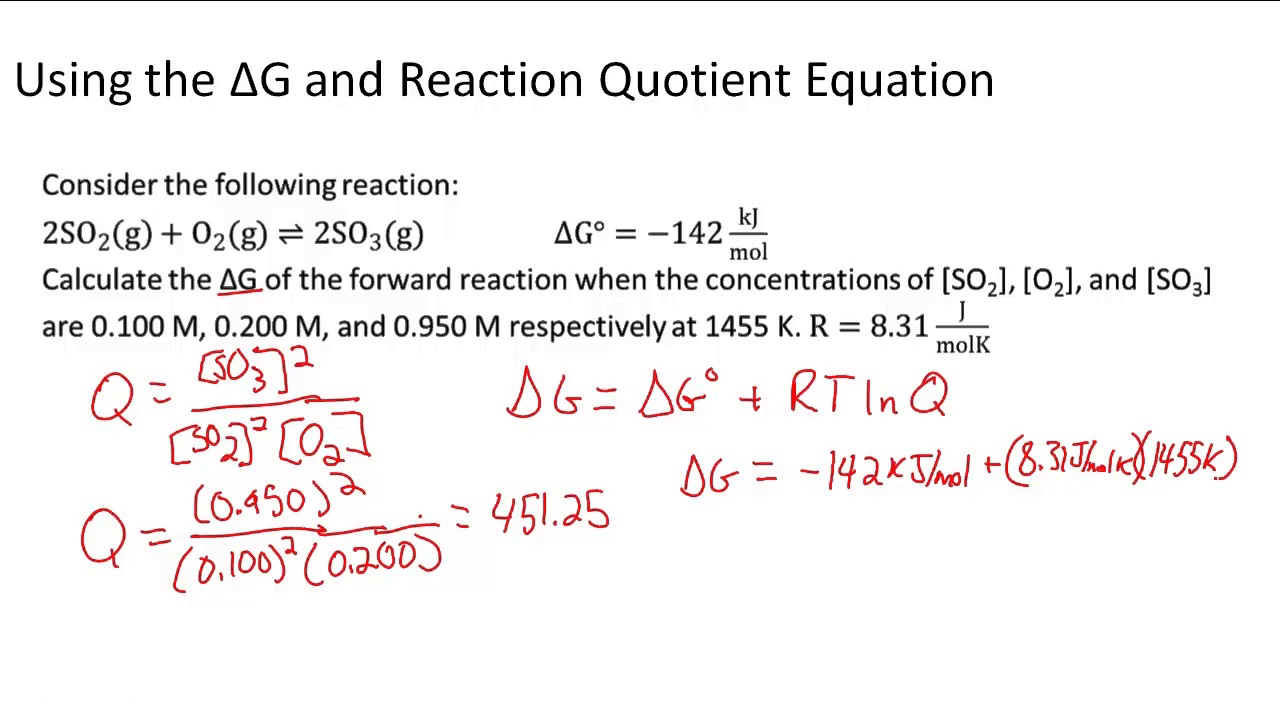
If we know the standard state free energy change g o for a chemical process at some temperature t we can calculate the equilibrium constant for the process at that temperature using the relationship between g o and k. The relationship between g o and the equilibrium constant for a chemical reaction is illustrated by the data in the table below. T is the temperature on the kelvin.
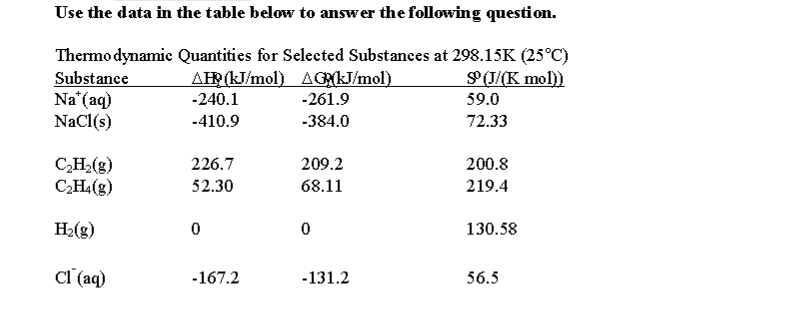
What Is The Free Energy For The Dissolution Of Solid Sodium Chloride In Water At 25c Socratic
socratic.org

Understanding The Difference Between Delta H And Delta S Concept Chemistry Video By Brightstorm
www.brightstorm.com