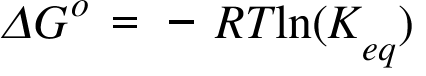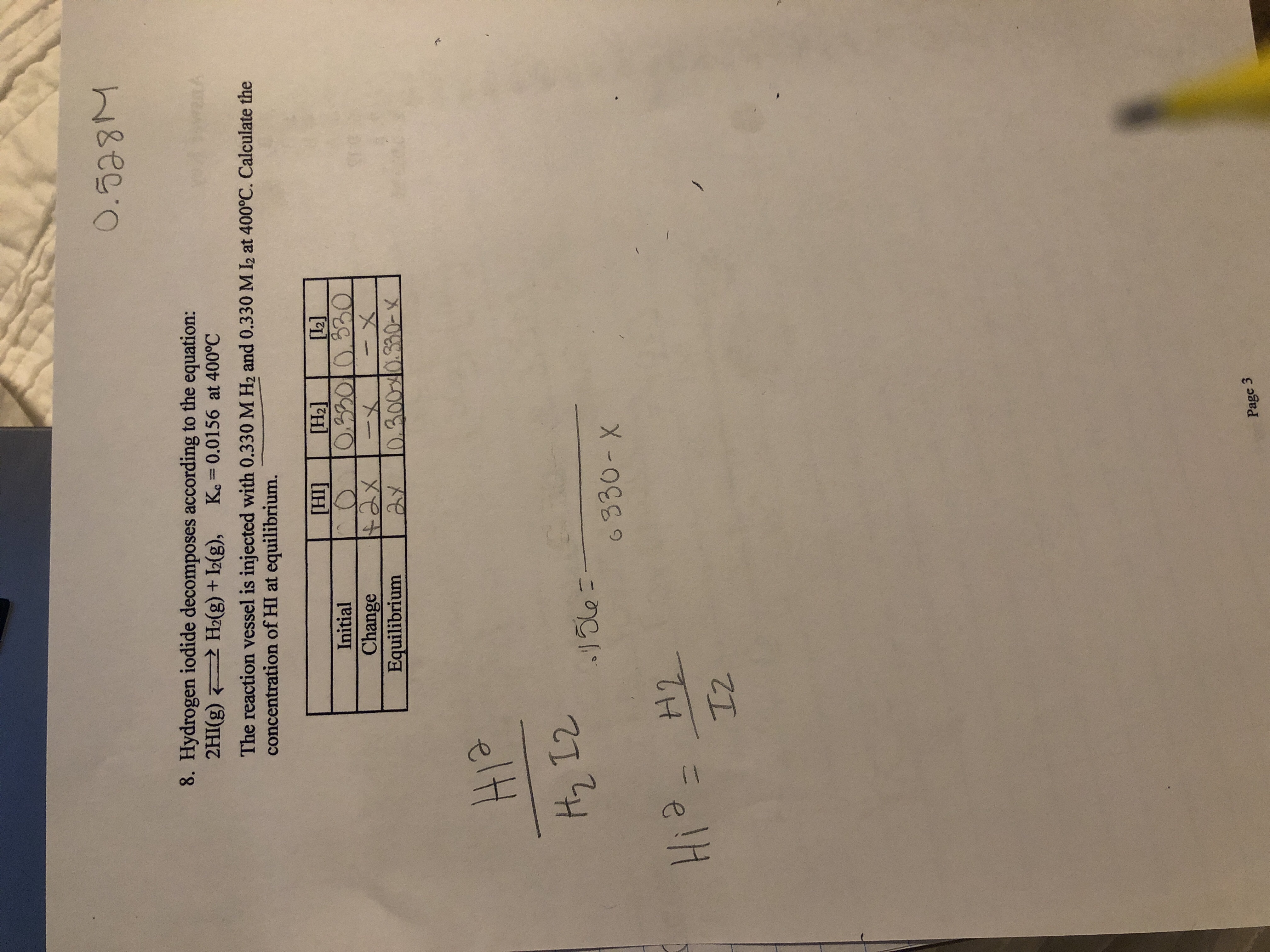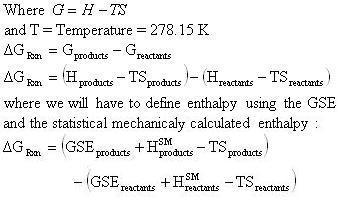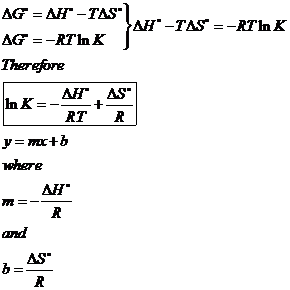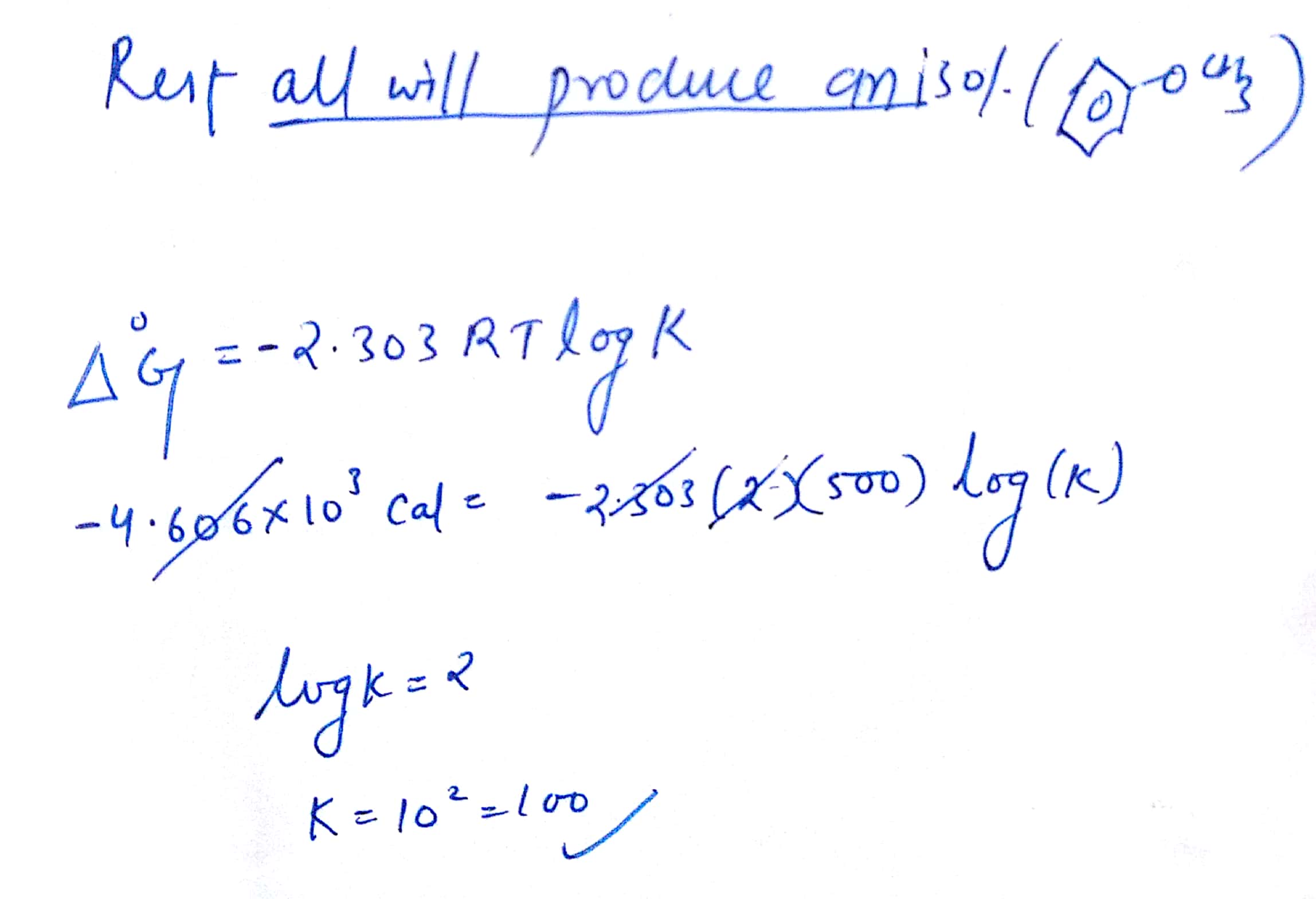Delta G Formula Equilibrium
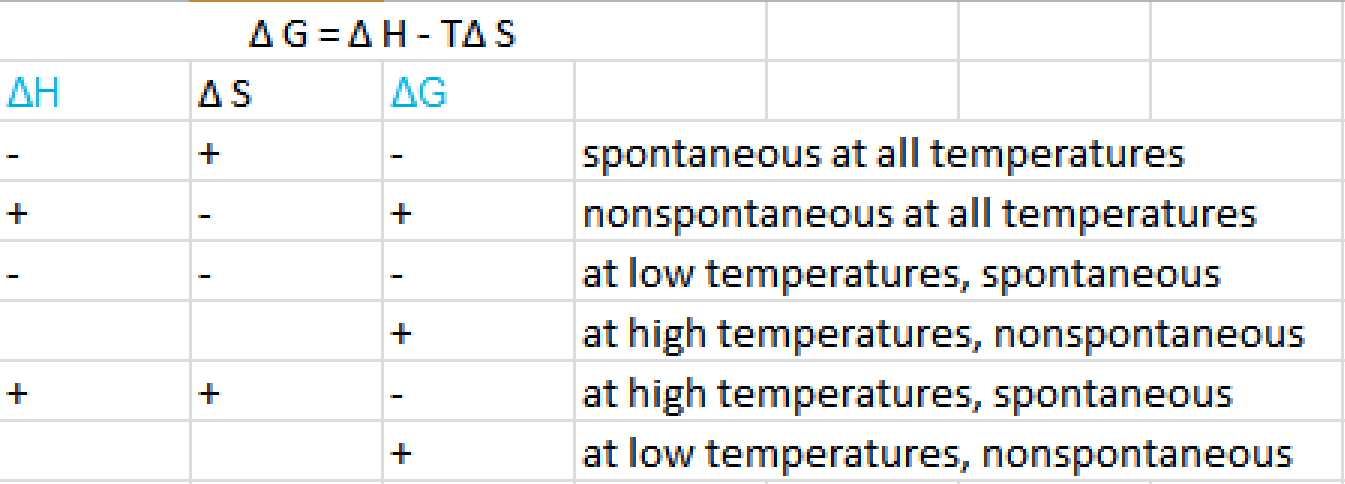
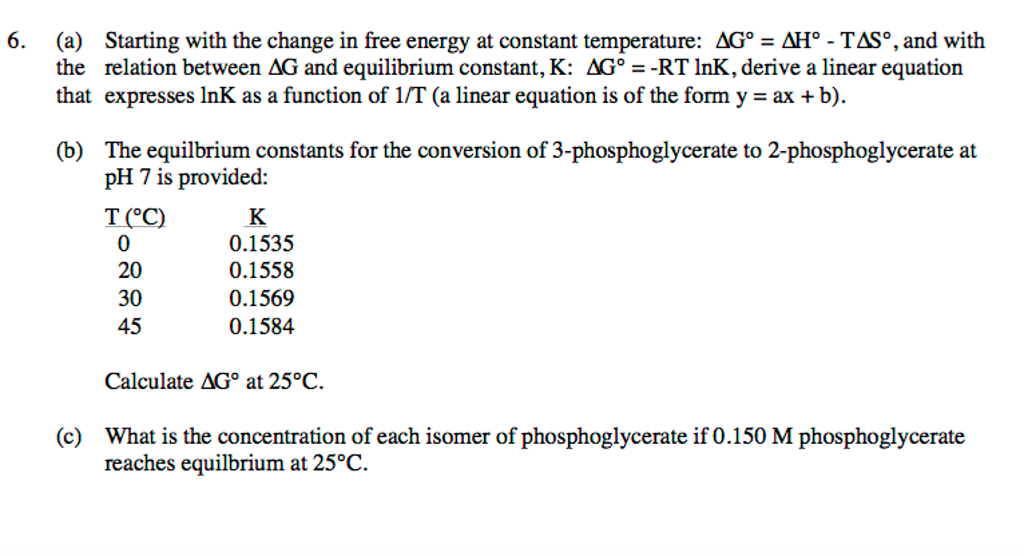
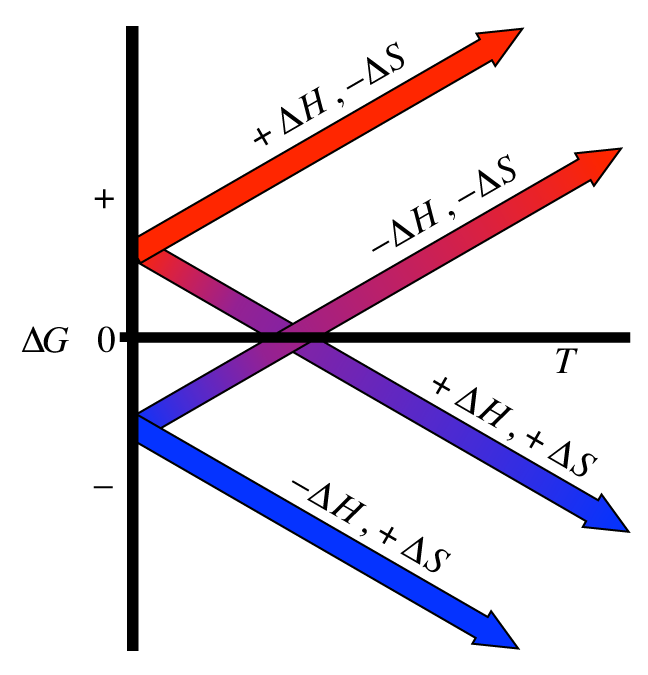
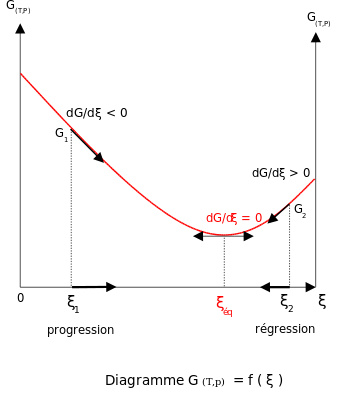
According to the second law of thermodynamics for systems reacting at standard conditions for temperature and pressure or any other fixed temperature and pressure there is a general natural tendency to achieve a minimum of the gibbs free energy.

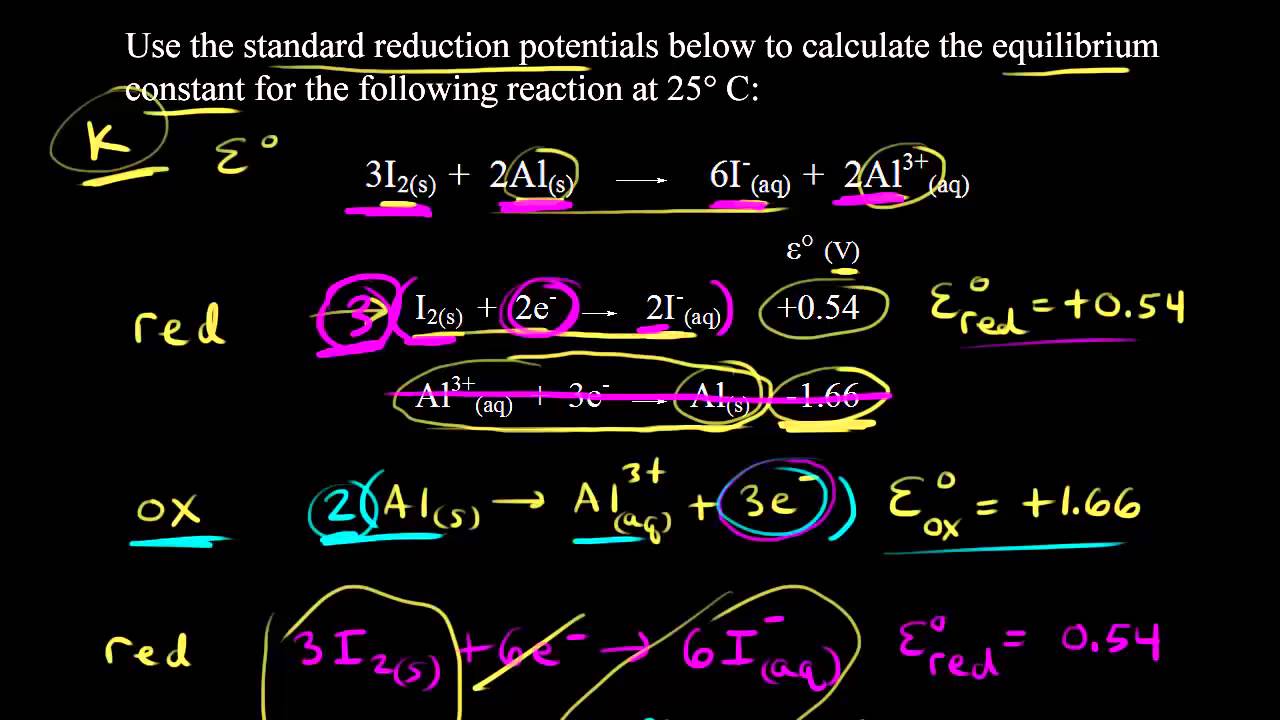
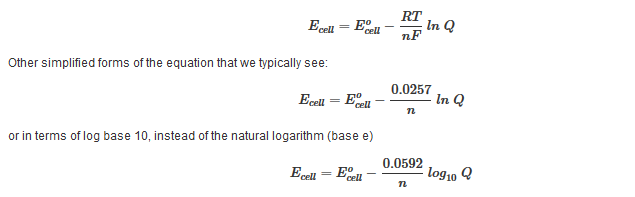
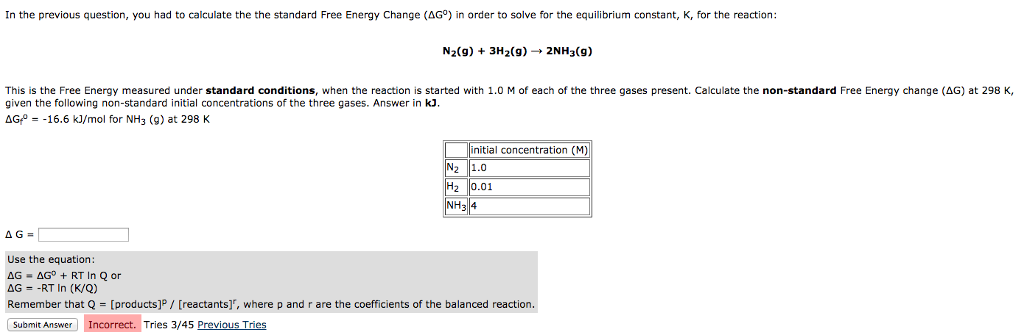
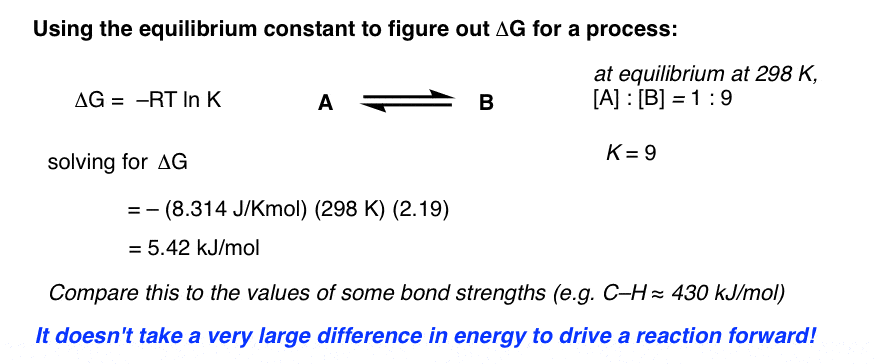
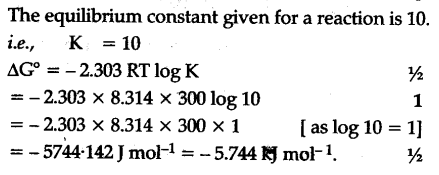
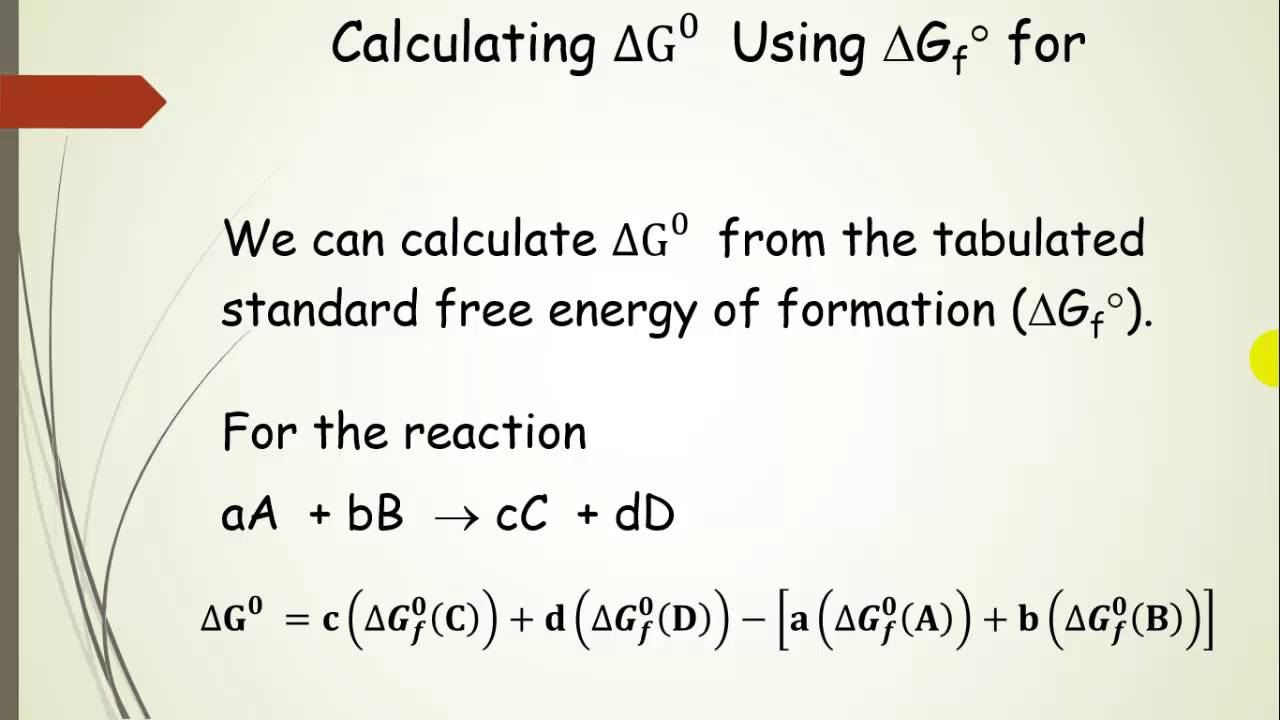
Delta g formula equilibrium. The reaction quotient is equal to the equilibrium constant. Rearrangement gives in this equation. Calculating an equilibrium constant from the free energy change.
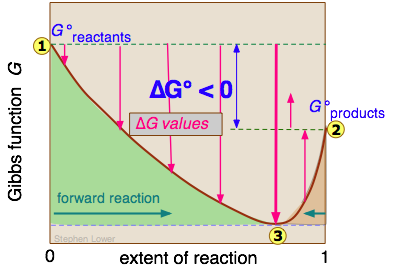
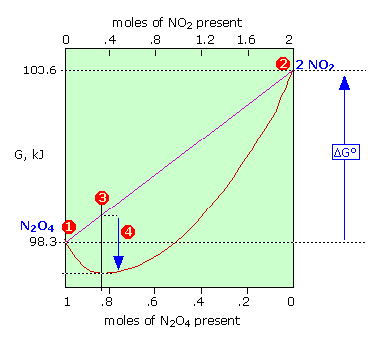
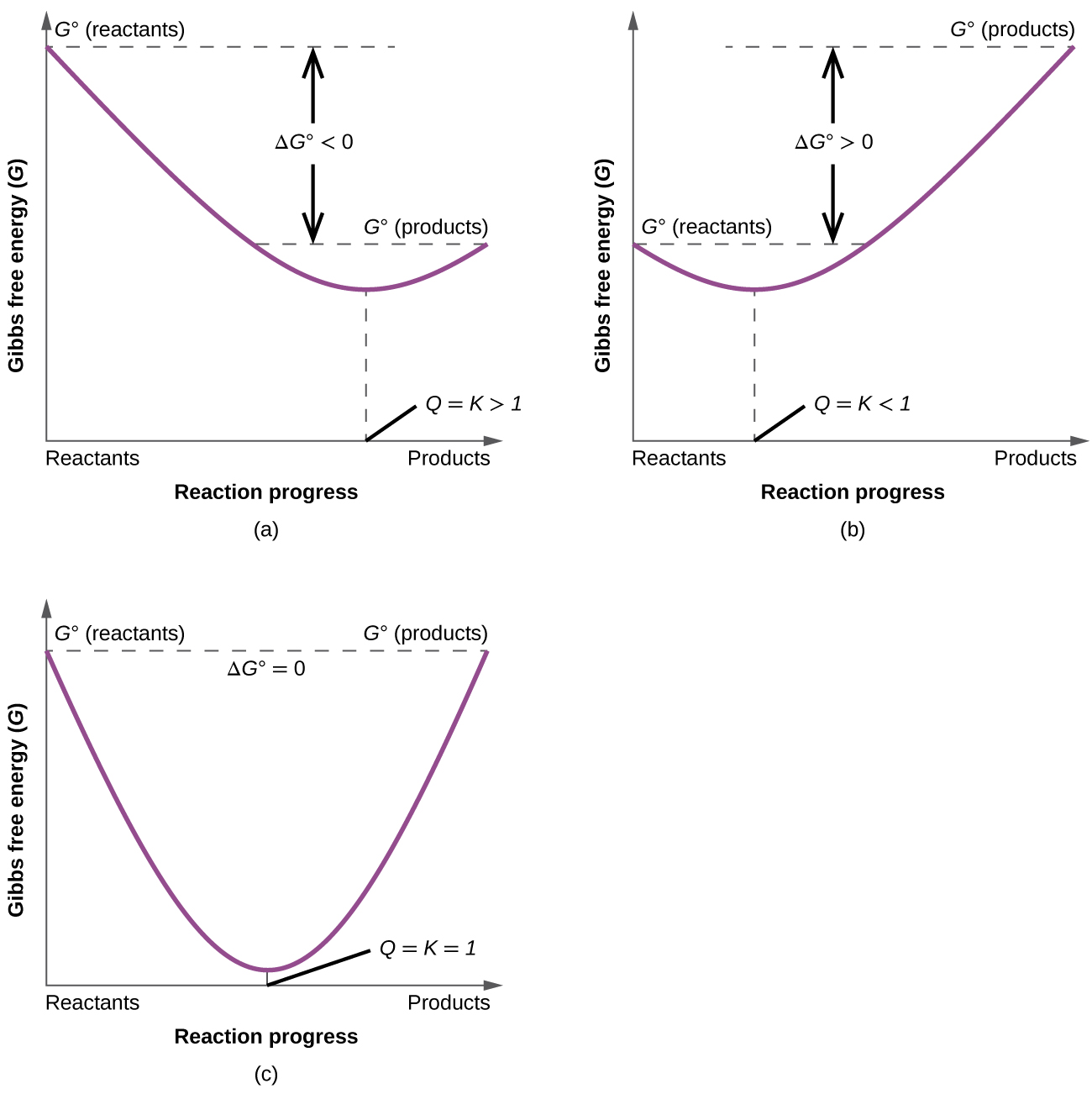
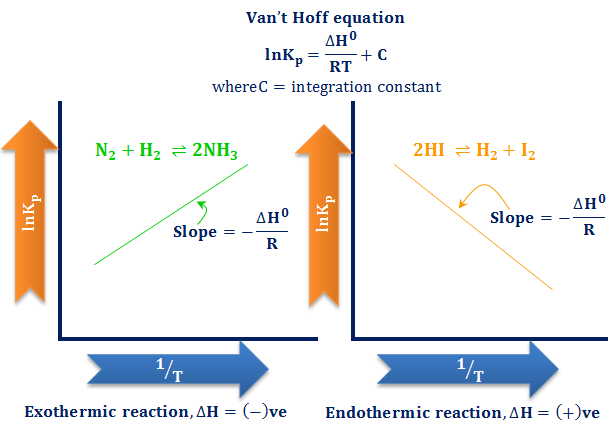
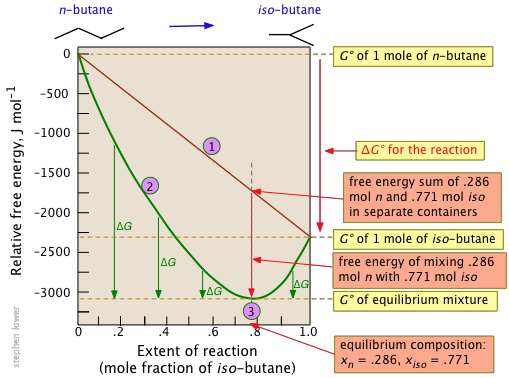
Lets plug that into our equation and see if thats true. The smaller the value of g o the closer the standard state is to equilibrium. If we know the standard state free energy change g o for a chemical process at some temperature t we can calculate the equilibrium constant for the process at that temperature using the relationship between g o and k.
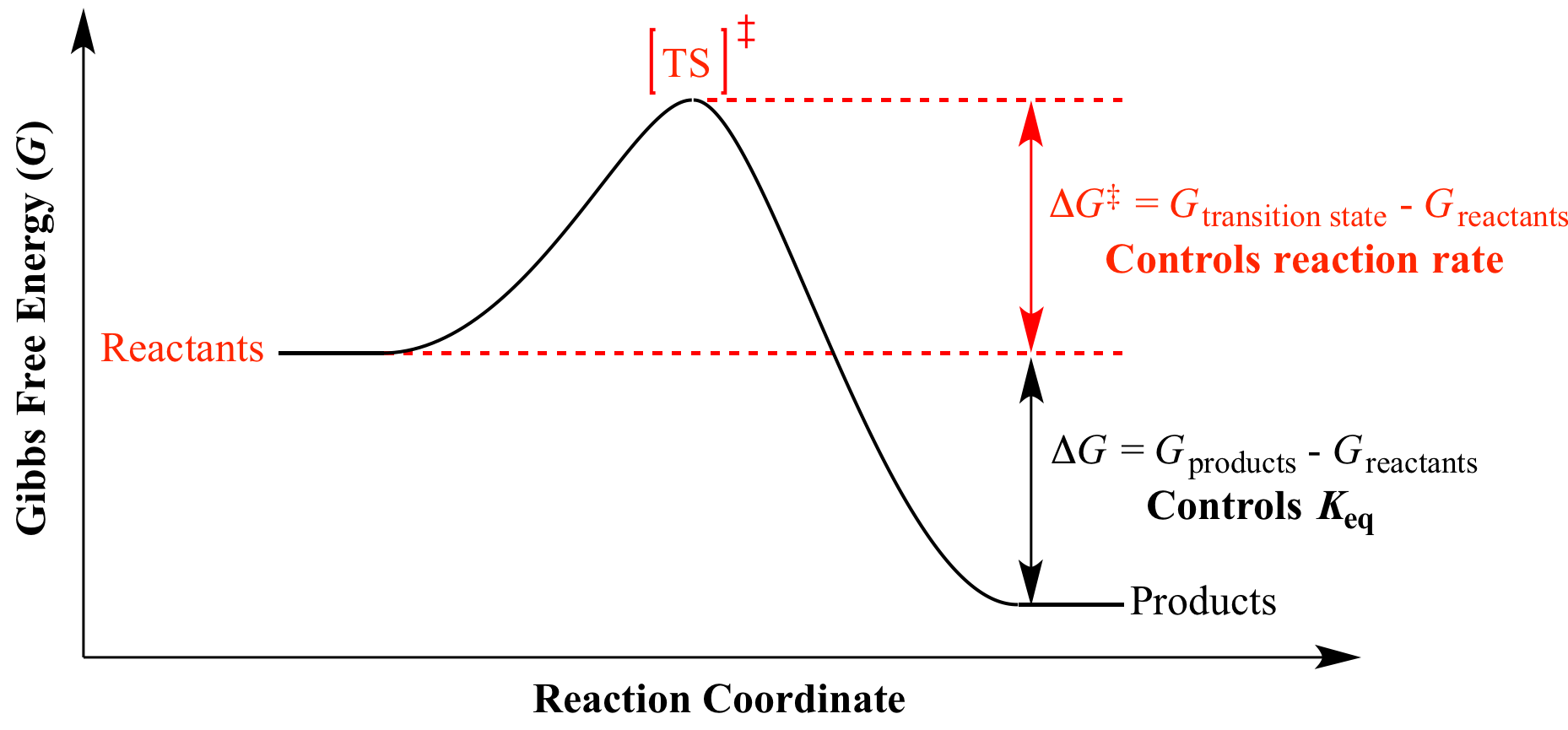
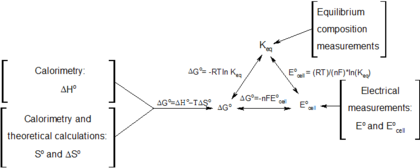
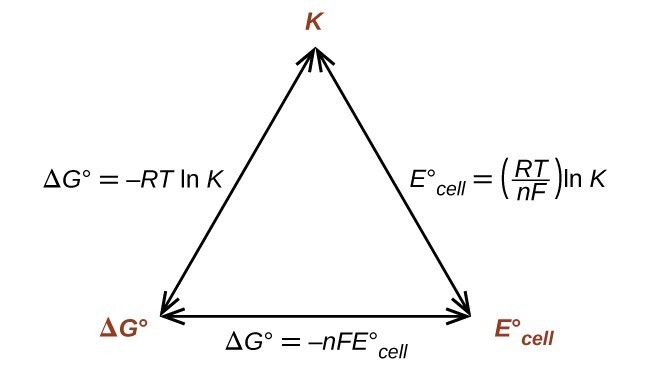
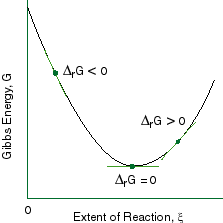
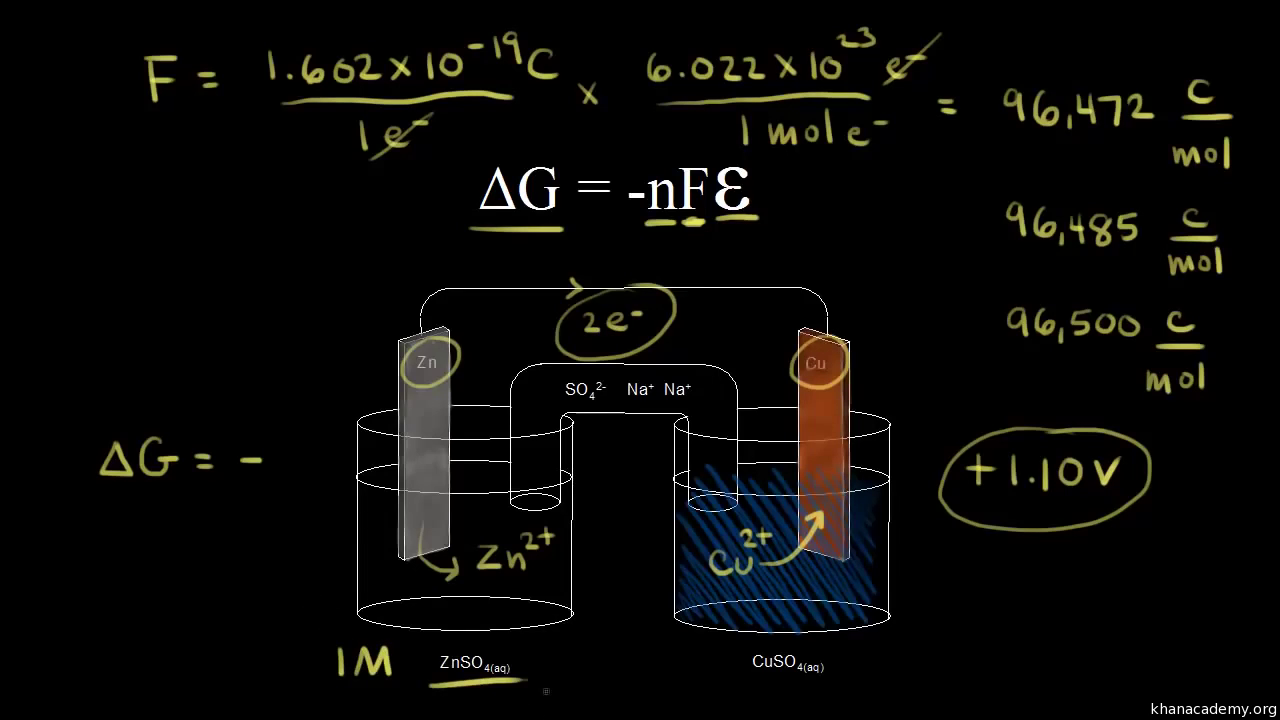
A quantitative measure of the favorability of a given reaction at constant temperature and pressure is the change dg sometimes written delta g. It is related to k at the equilibrium temp since then delta g is 0. 196 reduction potentials and the relationship between cell potential delta g and the equilibrium constant 197 electrolytic cells 198 electrolysis calculations.
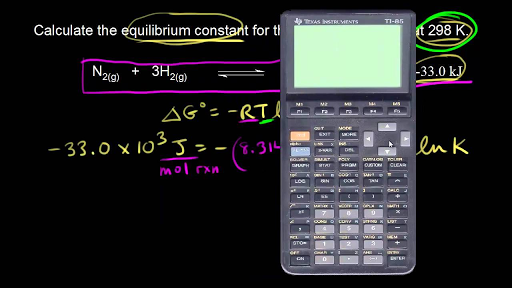
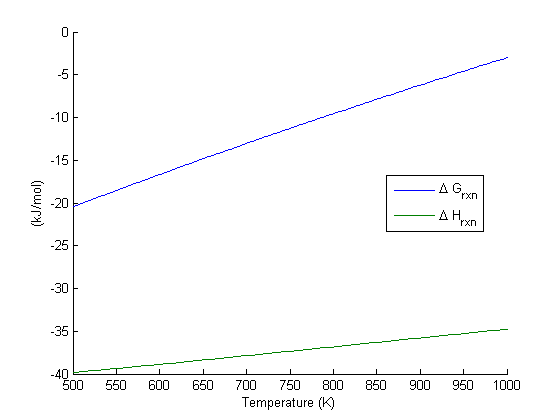
We have delta g is equal to delta g zero negative 330 times 10 to the third plus. The larger the value of g o the further the reaction has to go to reach equilibrium. 25 c 1 atm.
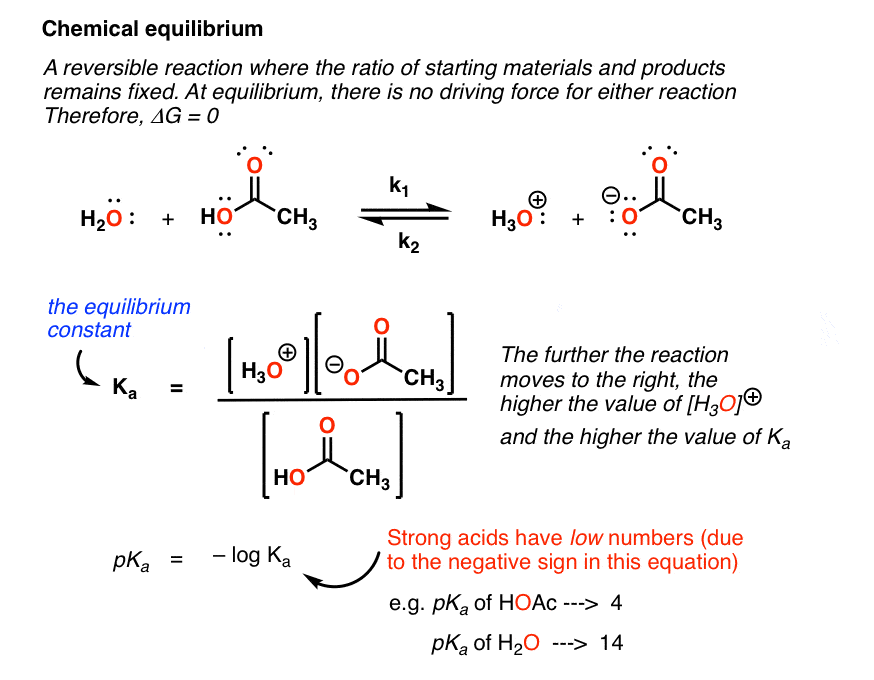
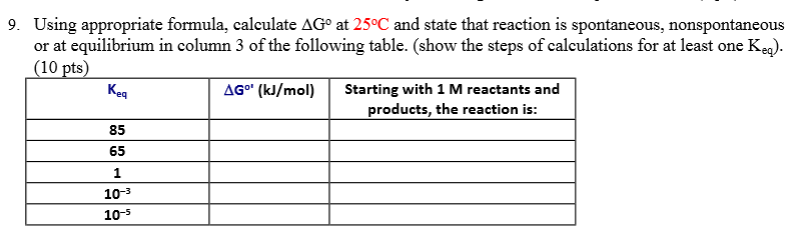
We know already that delta g should be equal to zero at equilibrium. The relationship between g o and the equilibrium constant for a chemical reaction is illustrated by the data in the table below. So equilibrium delta g should be equal to zero.
But delta g naught is the delta g at standard condition. This means of course that if the total gibbs gibbs energy g of a mixture of reactants and products goes through a minimum value as the composition changes then all net change will cease the reaction system will be in a state of chemical equilibriumyou will recall that the relative concentrations of reactants and products in the. Values of g o and k for common reactions at 25 o c.
So delta g naught is constant for a given reaction.
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gctbuanynjvalrcfljiodwgh U6rpw0diiiwxqow Gosk78pnvxo Usqp Cau
encrypted-tbn0.gstatic.com

Gibbs Free Energy Changes Equation Calculations Reaction Feasibility Extraction Of Metals Cell Emf Gce A Level Chemistry Revision Notes
www.docbrown.info

Find Out The Value Of Equilibrium Constant For The Following Reaction At 298 K 2nh 3 G Co Youtube
www.youtube.com