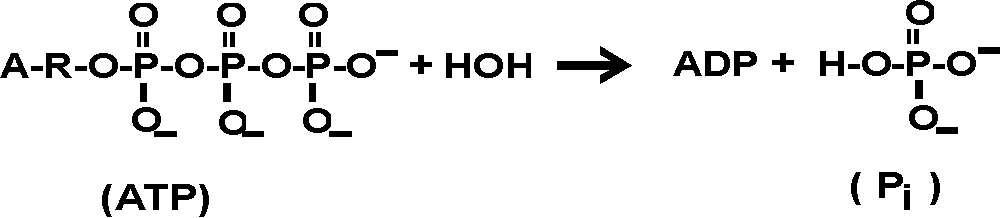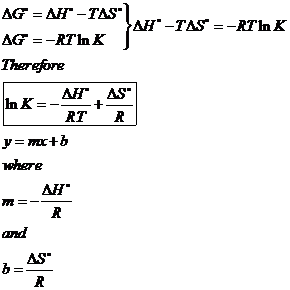Delta G Formula Chemistry
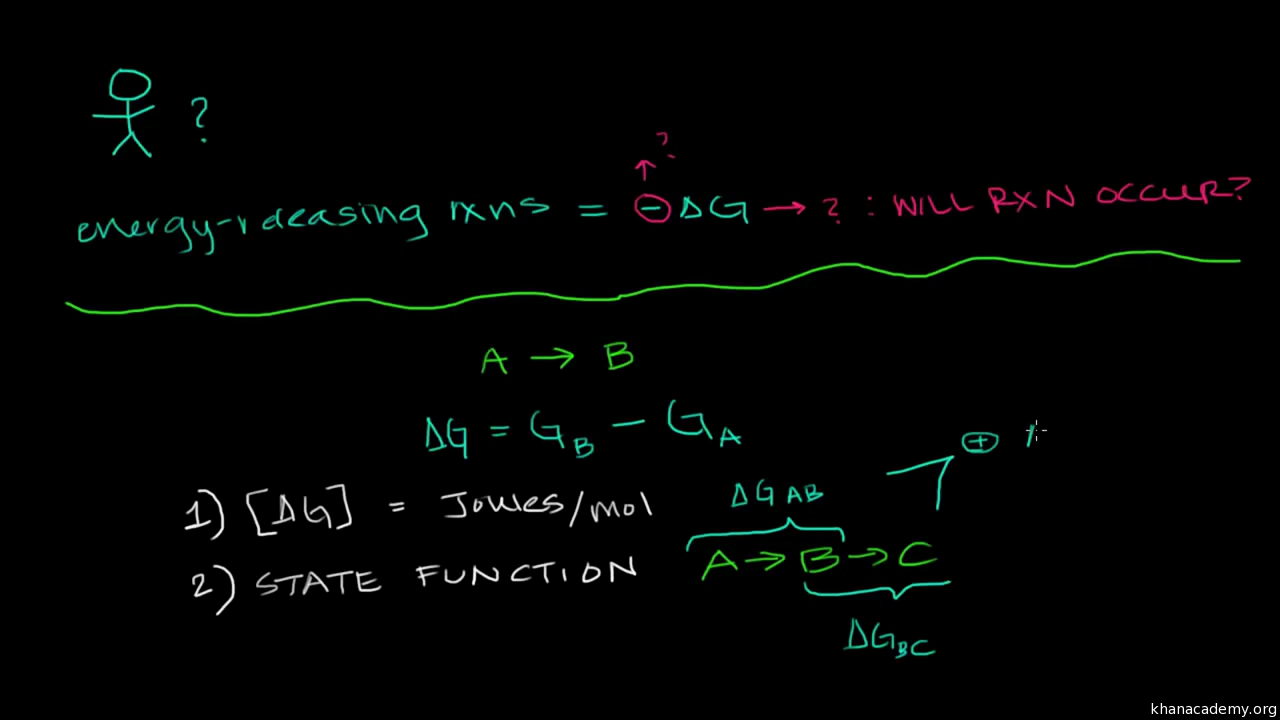
So over here delta g is going to be less than zero.

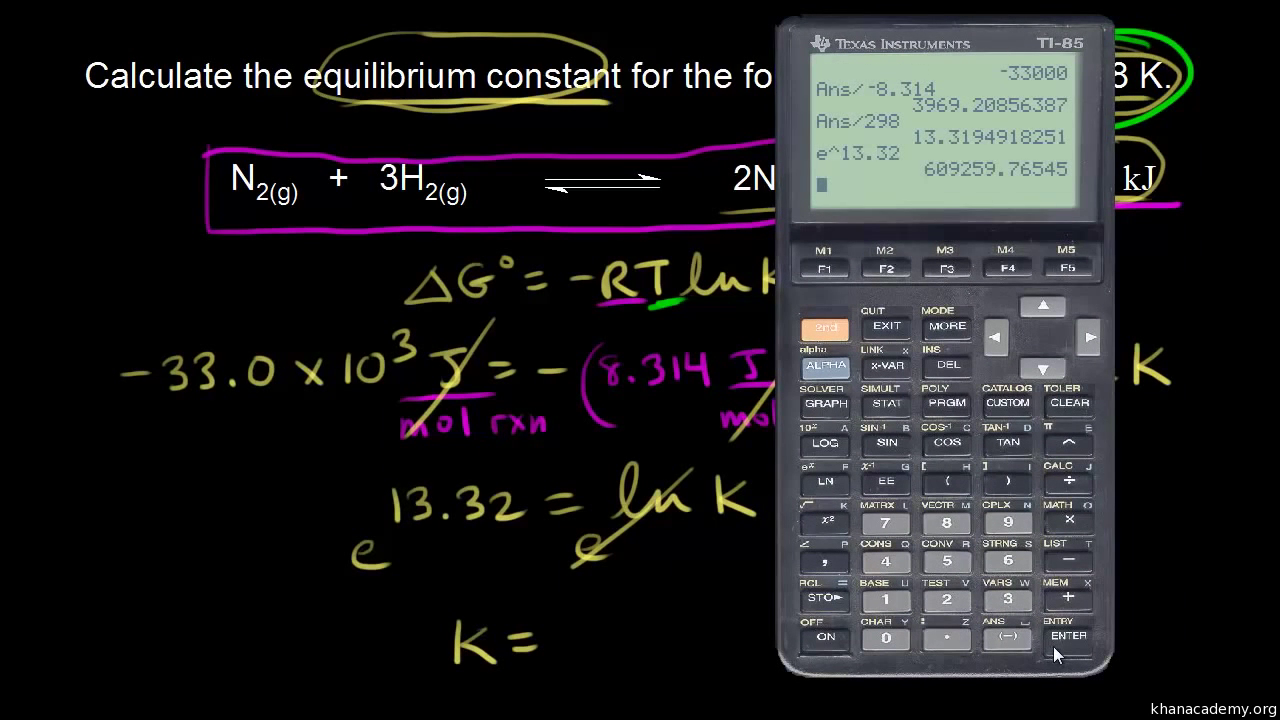
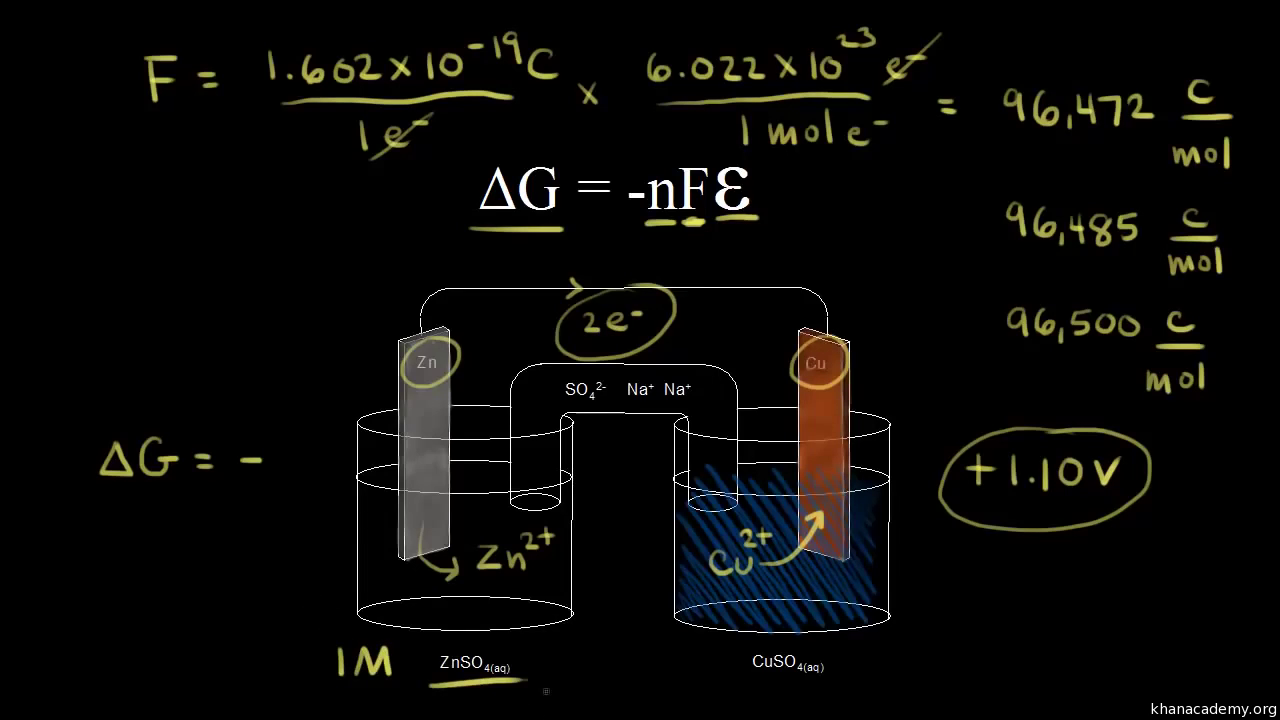
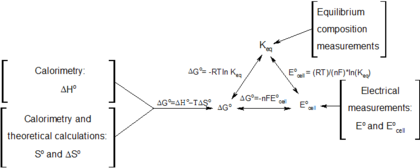
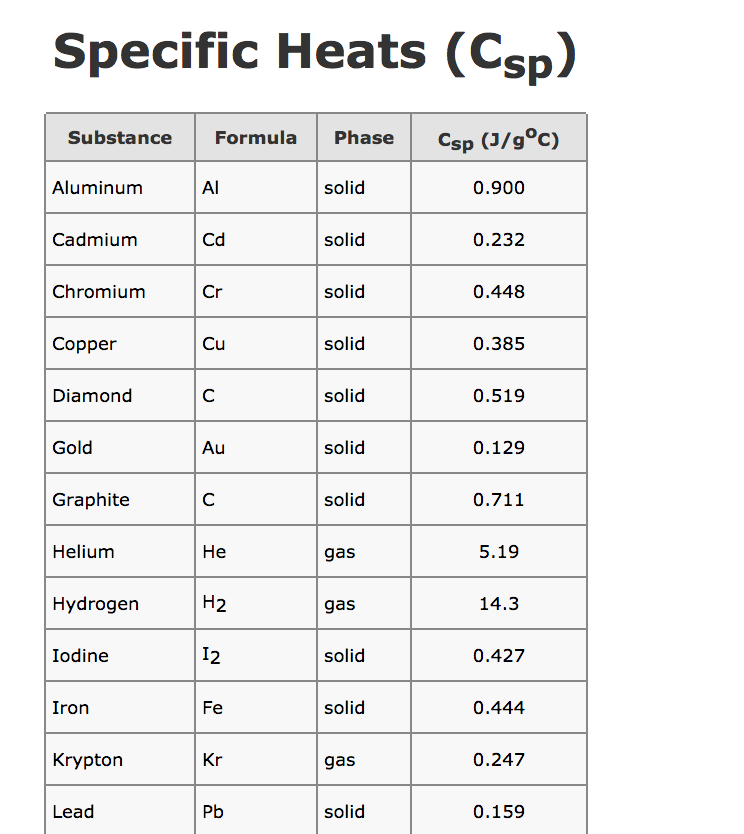
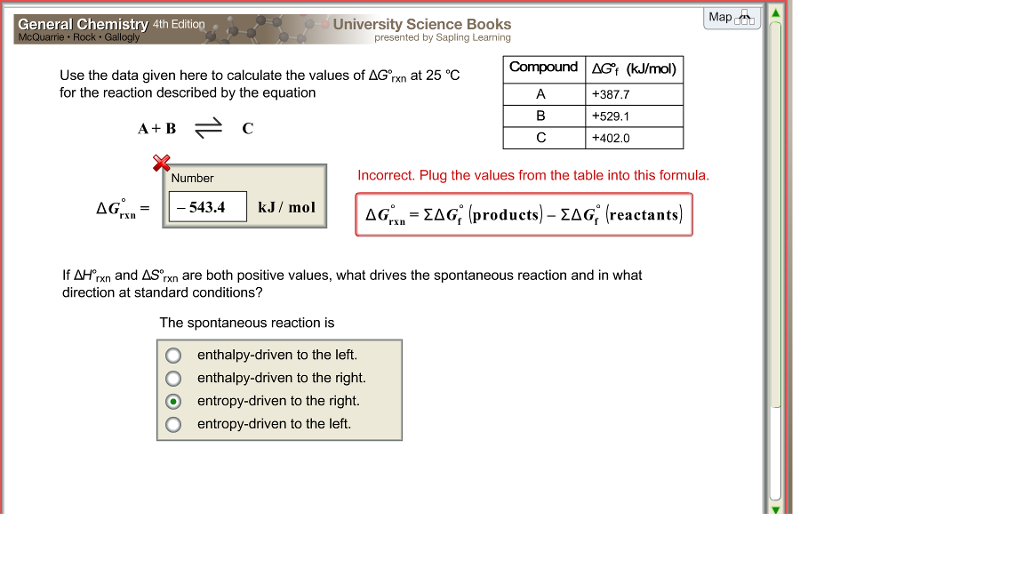
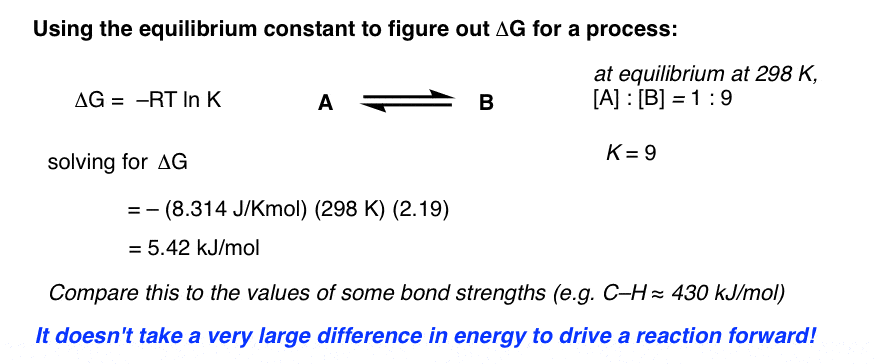
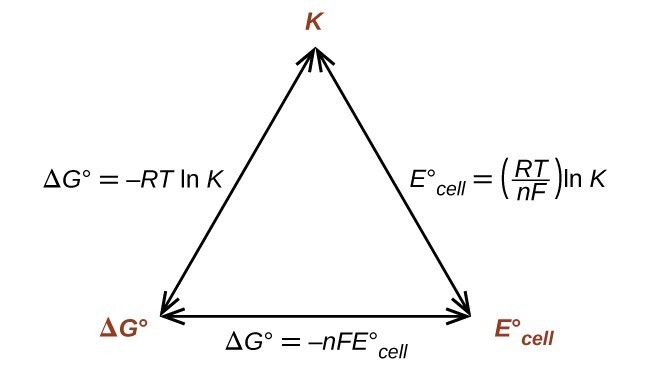
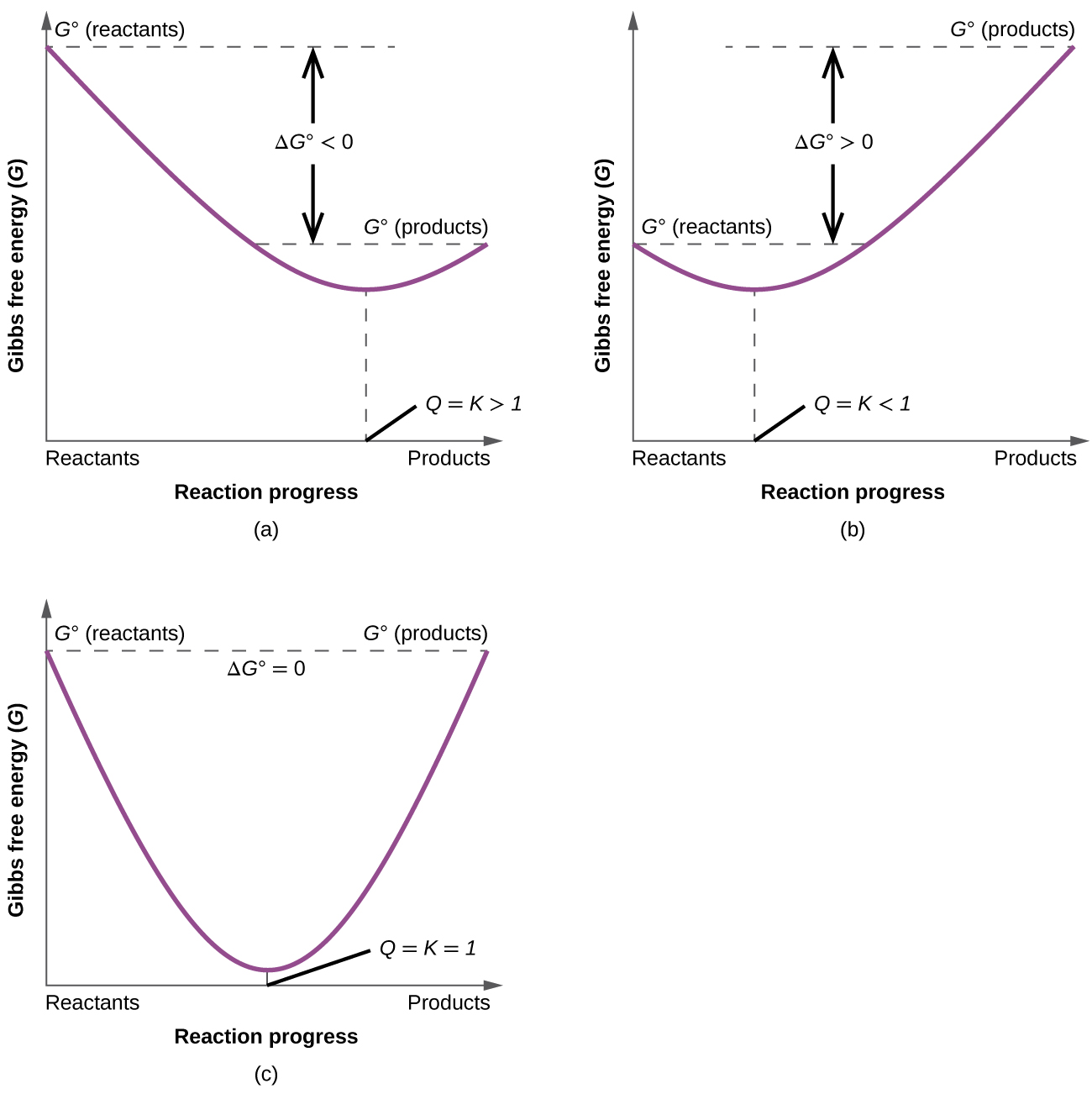
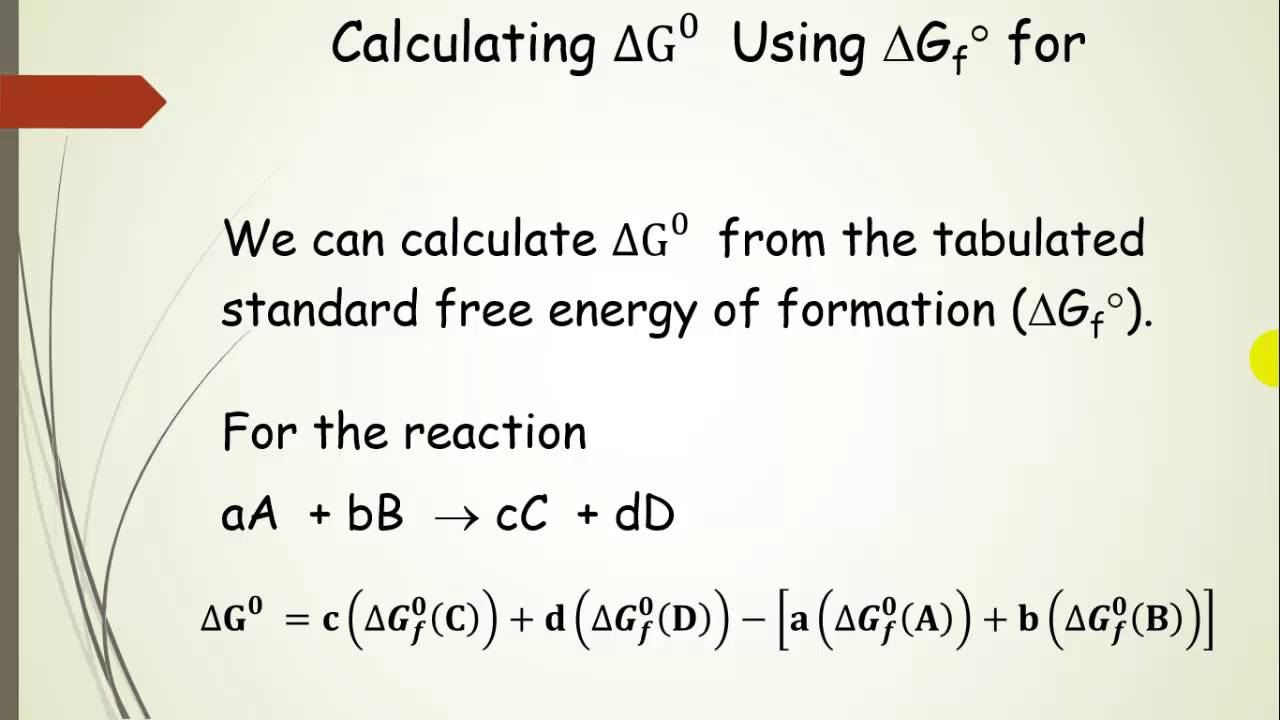
Delta g formula chemistry. Consequently there must be a relationship between the potential of an electrochemical cell and deltag. Consider the two equations that deal with delta g g. Values of g o and k for common reactions at 25 o c.
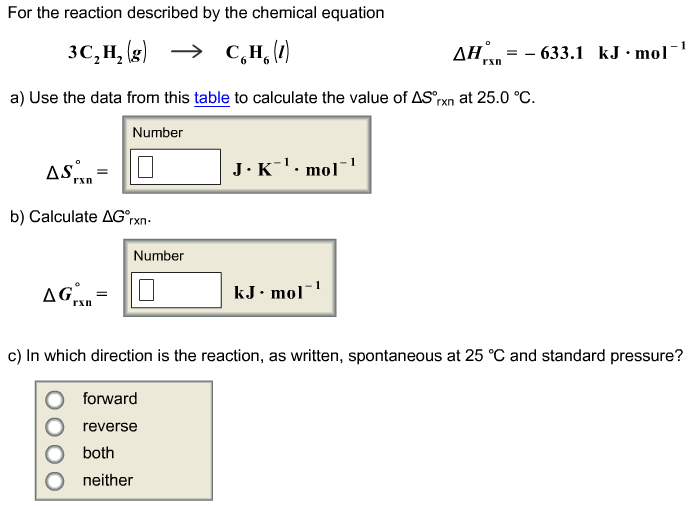
A quantitative measure of the favorability of a given reaction at constant temperature and pressure is the change dg sometimes written delta g. D s is in the units of joules per kelvin j k. Click to learn more.
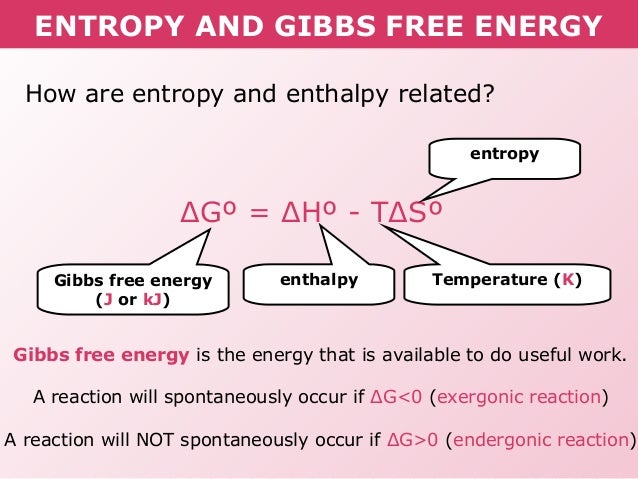
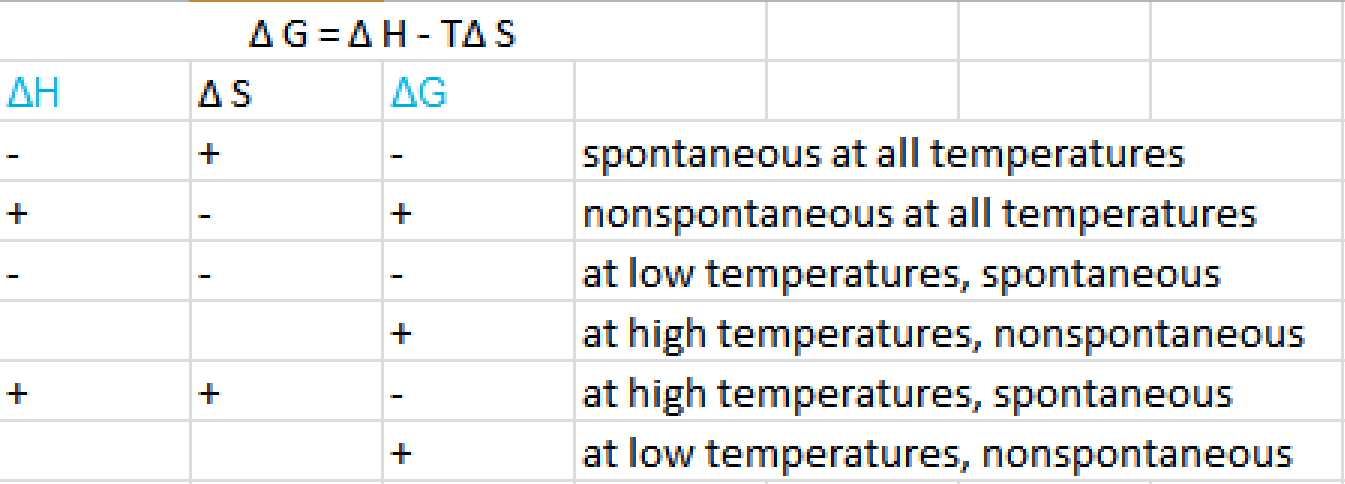
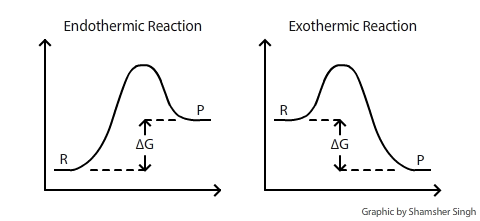
In chemistry delta g refers to the change in gibbs free energy of a reaction. 183 gibbs free energy and the relationship between delta g delta h and delta s. Gibbs free energy refers to the energy in a chemical reaction that can be used to do work.
D h is in the units of joules j. General chemistry quizzes practice exams study guides and more. In the previous equation.
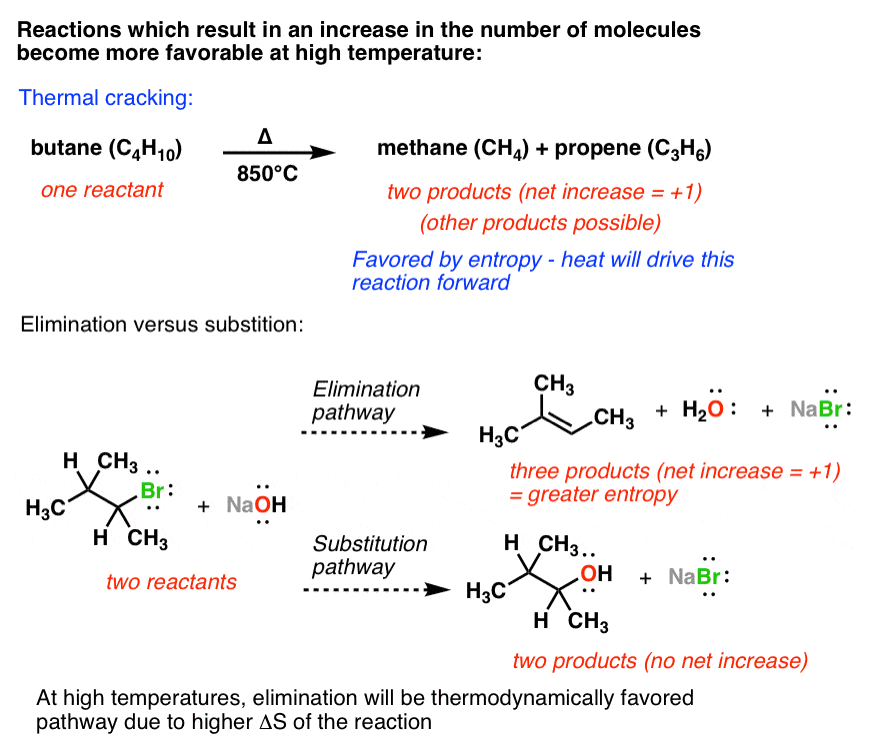
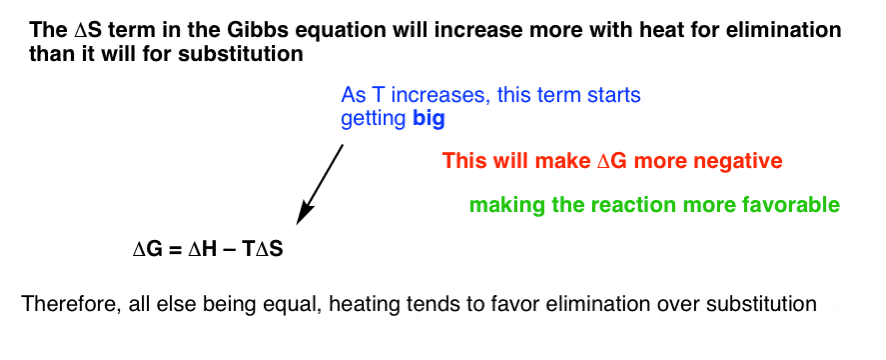
As such i think that knowledge of it and the consequences associated with it are. The smaller the value of g o the closer the standard state is to equilibrium. Even if delta h is greater than zero even if this is positive if delta s is greater than zero and t is high this thing is going to become especially with the negative sign here this is going to overwhelm the enthalpy and the change in enthalpy and make the whole expression negative.
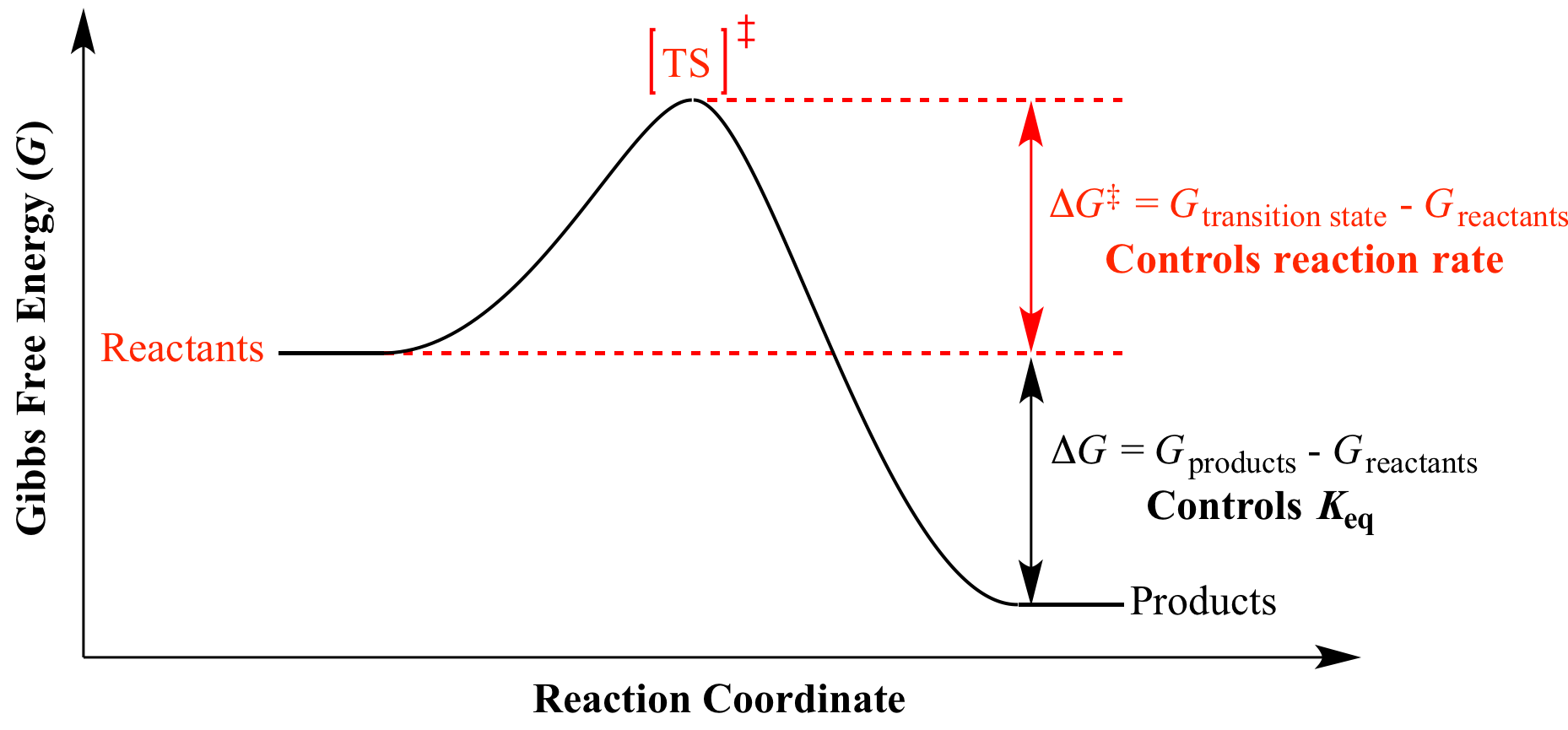
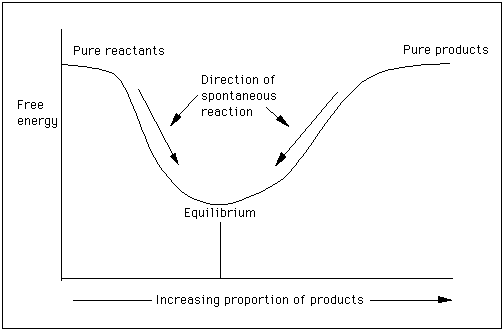
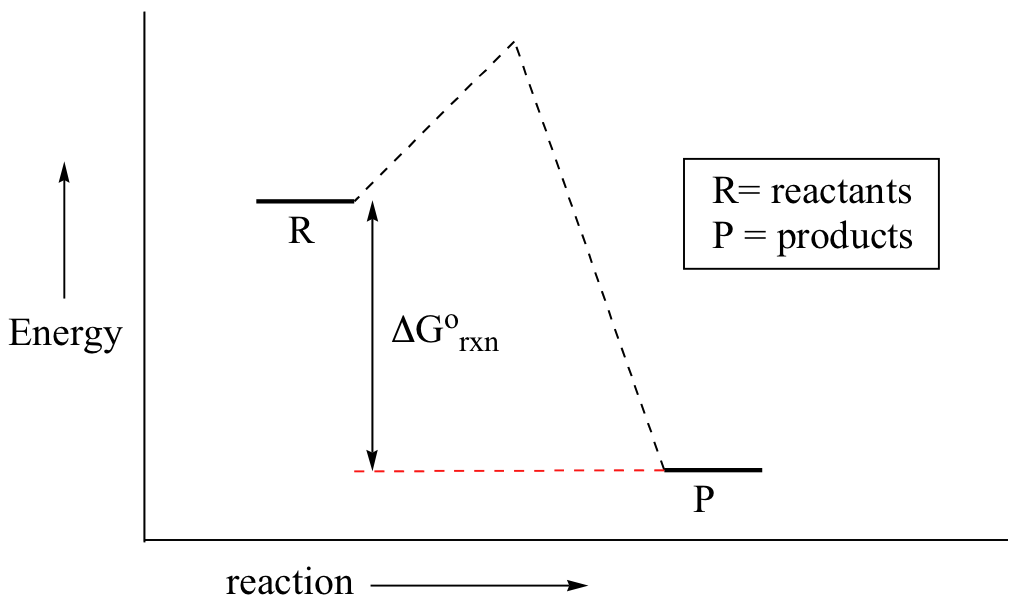
The change in free energy deltag is also a measure of the maximum amount of work that can be performed during a chemical process dg wmax. According to the second law of thermodynamics for systems reacting at standard conditions for temperature and pressure or any other fixed temperature and pressure there is a general natural tendency to achieve a minimum of the gibbs free energy. However again because all of these are linked to chemistry and because chemistry likes to measure everything per mole all of the variables above but temperature may also have attached to them per mole mol.
T is in the units of kelvin k. One of the changes was to remove equation 2 below from the equations constants sheet. Delta h is the.
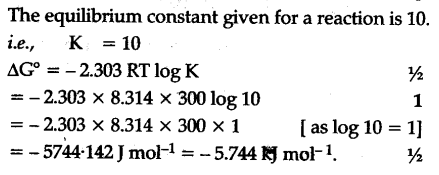
Gibbs free energy denoted g combines enthalpy and entropy into a single value. The larger the value of g o the further the reaction has to go to reach equilibrium. Since this post was originally written in january 2012 the ap exam has changed.
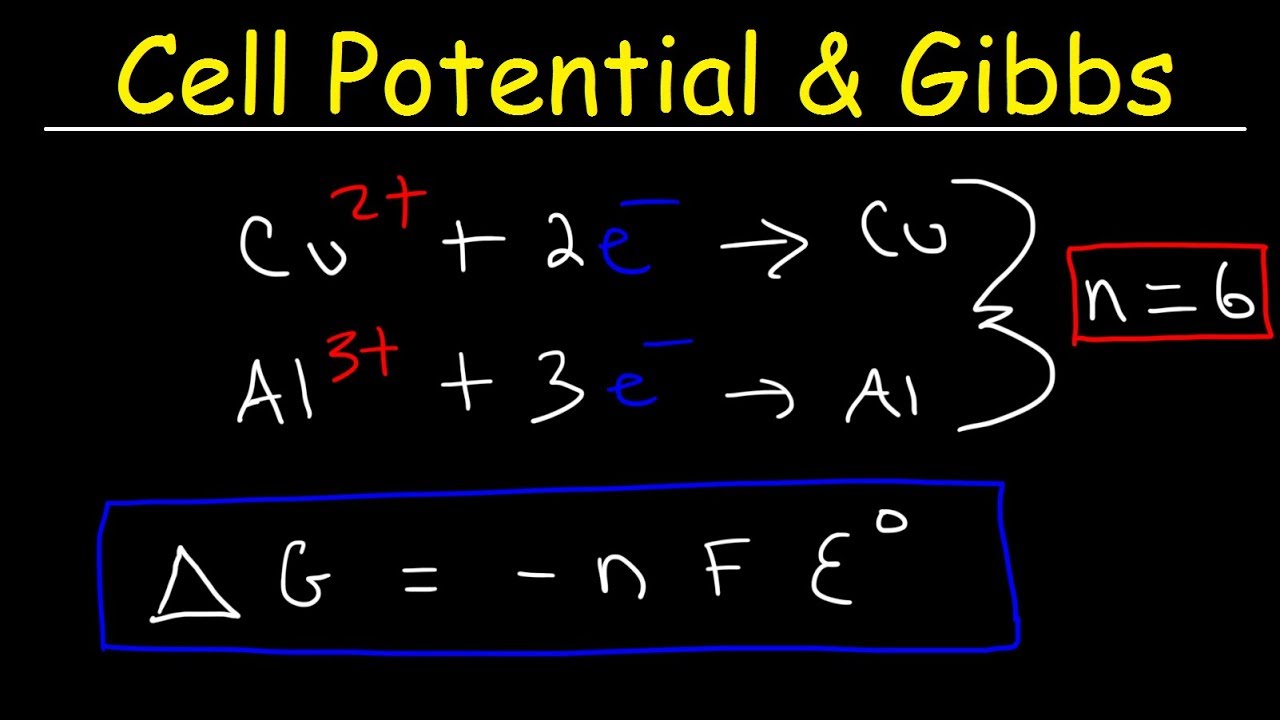
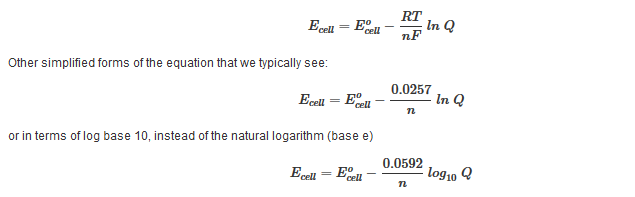
D g is in the units joules j. Deltag nfecell label2054. This relationship is as follows.

Is Anyone Familiar With The Derivation Of The Modified Gibbs Helmholtz Equation
www.researchgate.net

Gibbs Free Energy Changes Equation Calculations Reaction Feasibility Extraction Of Metals Cell Emf Gce A Level Chemistry Revision Notes
www.docbrown.info