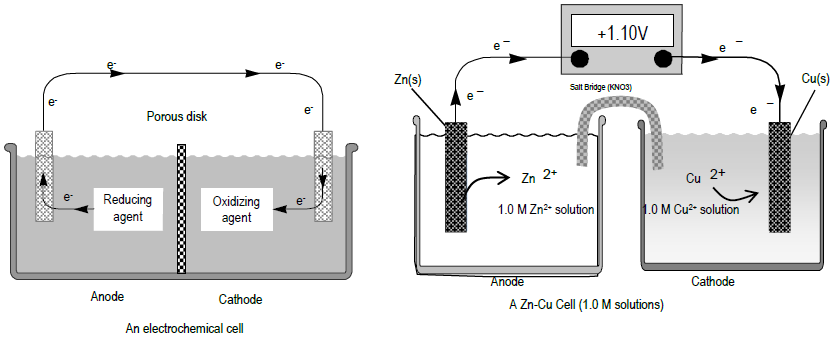
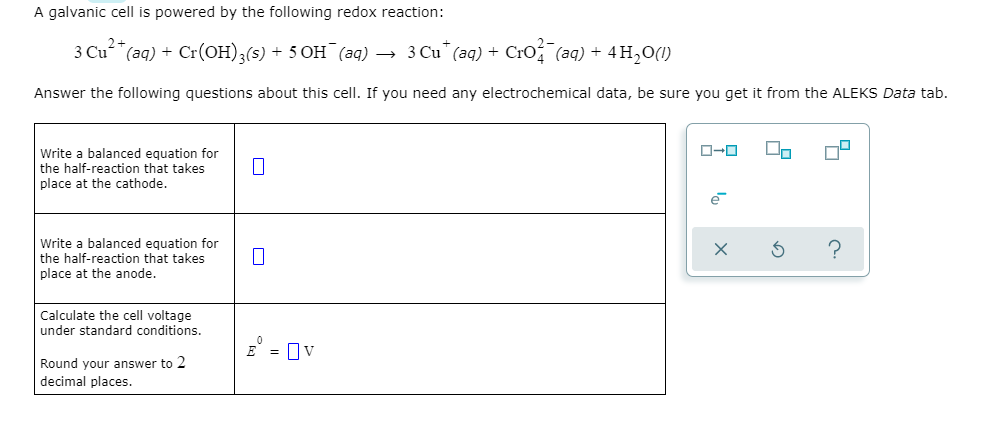
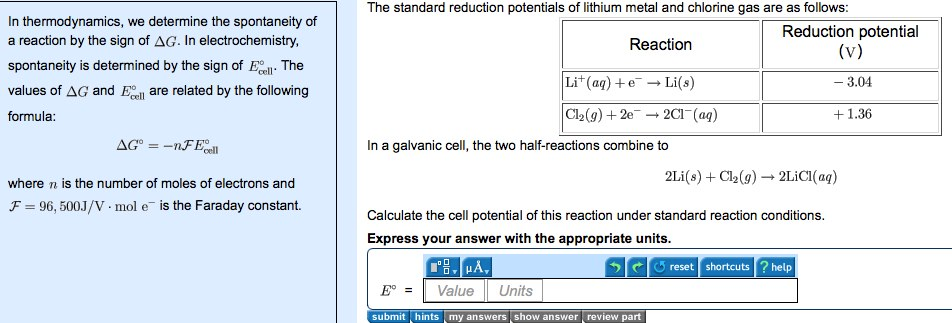
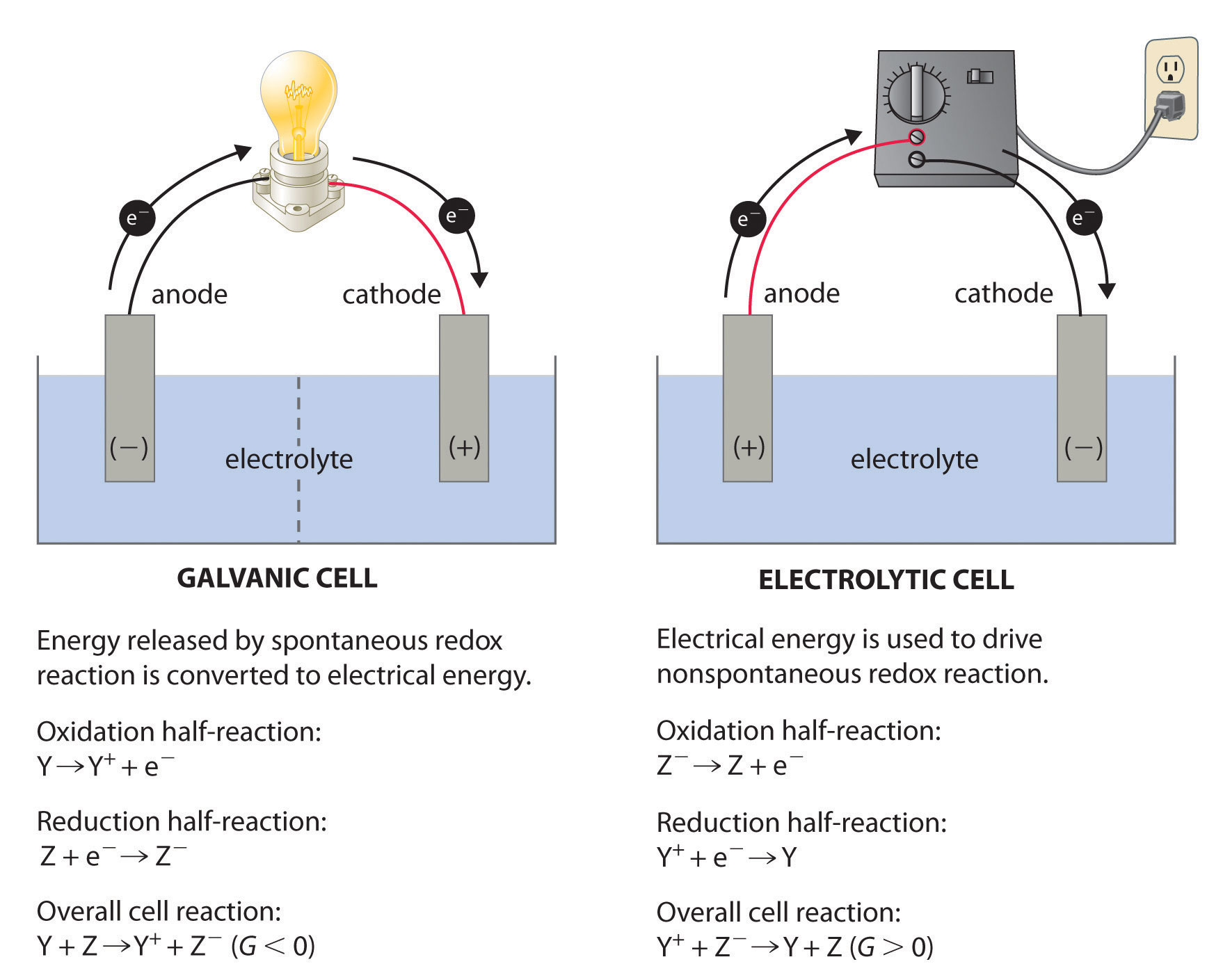
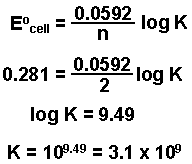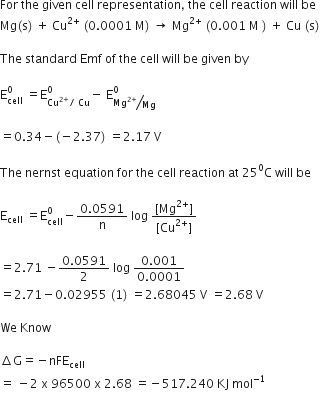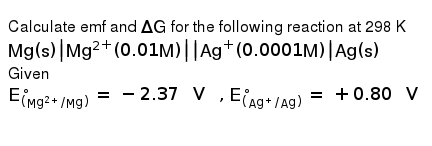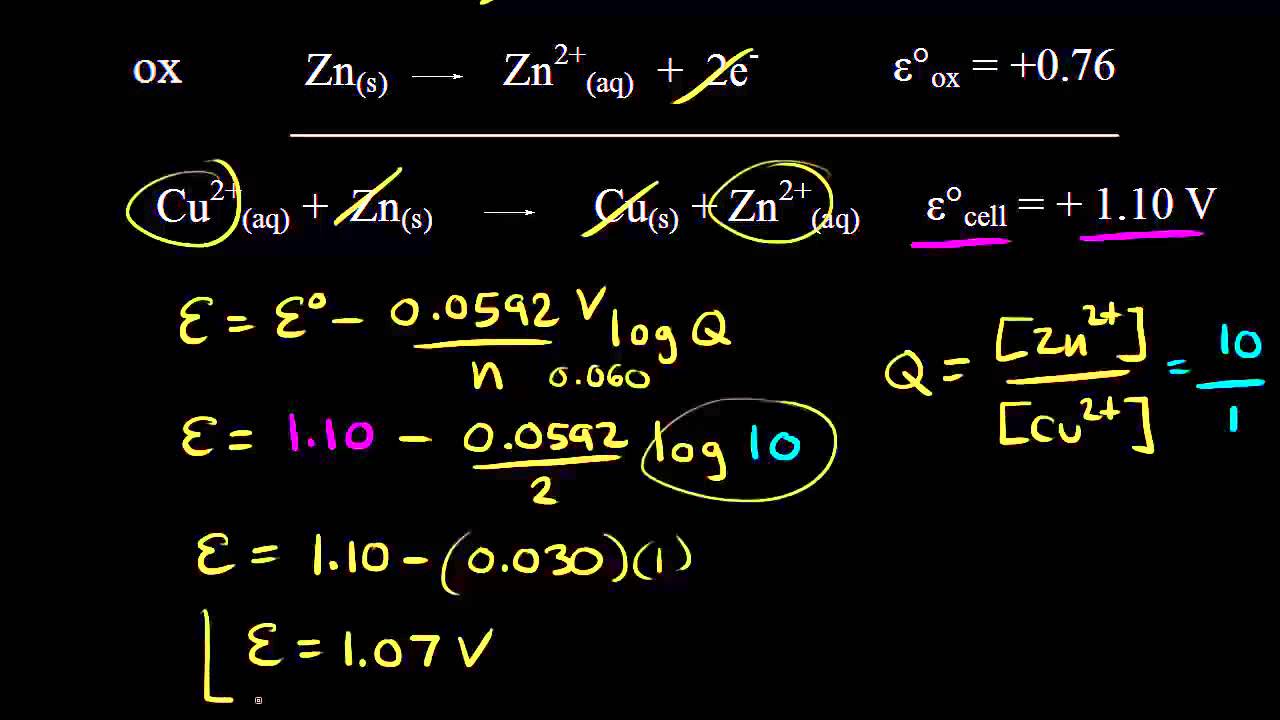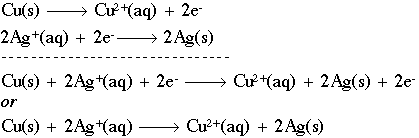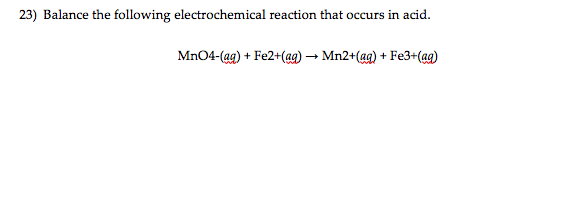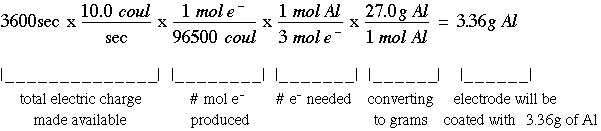Delta G Formula Electrochemistry
And we know for a spontaneous reaction delta g is negative.

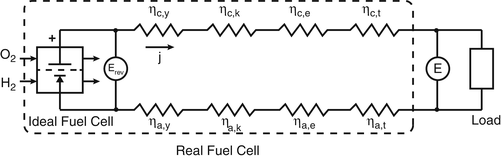
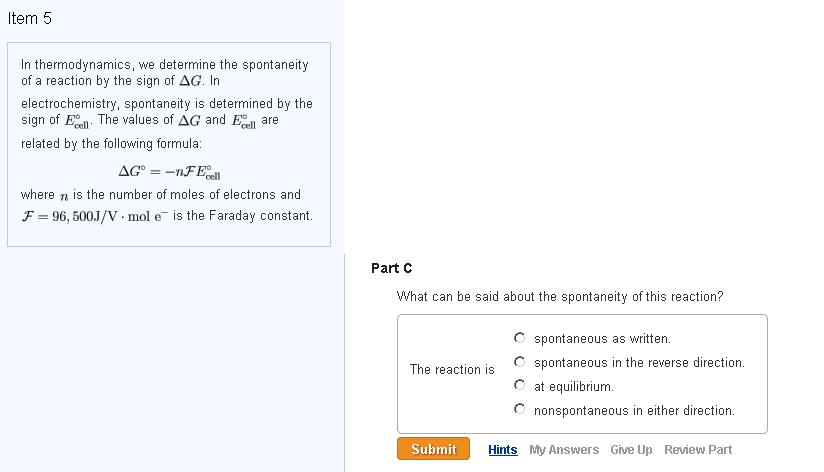
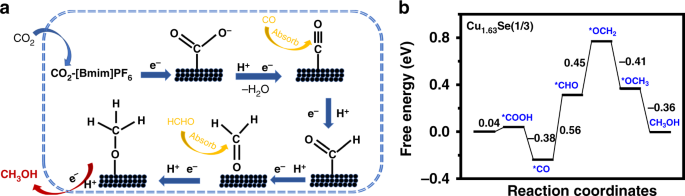
Delta g formula electrochemistry. So delta g is negative for our spontaneous redox reaction that we have in our voltaic cell. Deltag nfecello where. And in this one it should be 2 electrons if i am not mistaken.
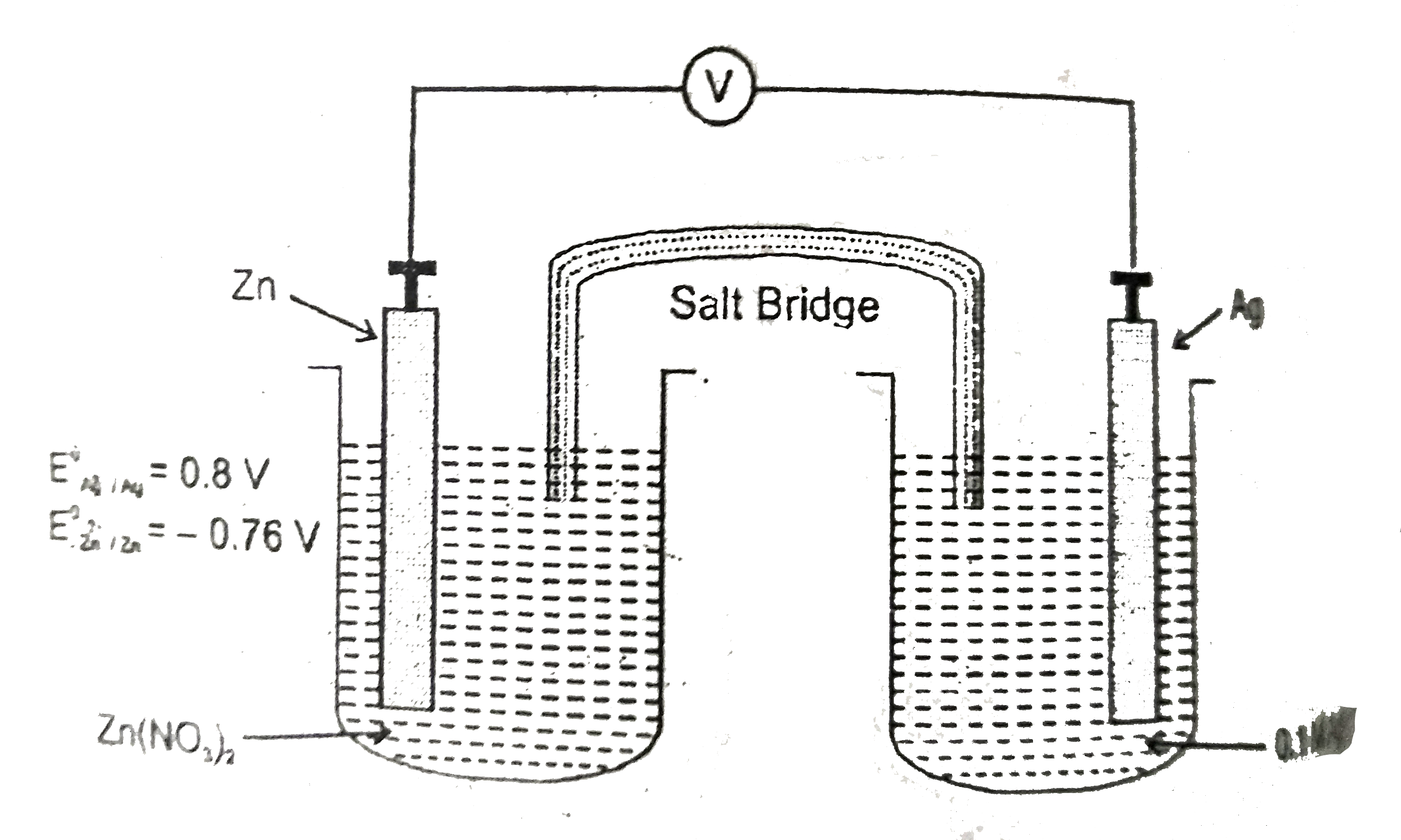
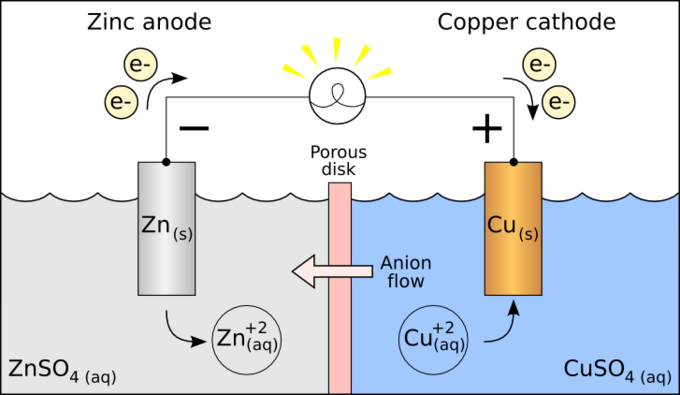
Electrochemistry is the branch of physical chemistry that studies the relationship between electricity as a measurable and quantitative phenomenon and identifiable chemical change with either electricity considered an outcome of a particular chemical change or vice versathese reactions involve electric charges moving between electrodes and an electrolyte or ionic species in a solution. Latexdelta textg delta textgtexto textrt times textln textqlatex here dg is the change in gibbs free energy t is absolute temperature r is the gas constant and q is the reaction quotient. You cannot apply the deltag equation to a single electrode potential.
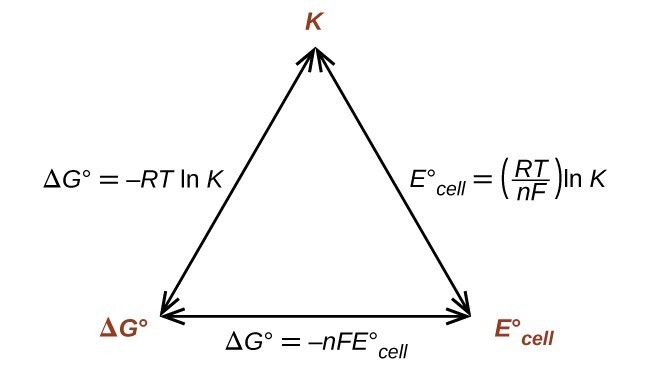
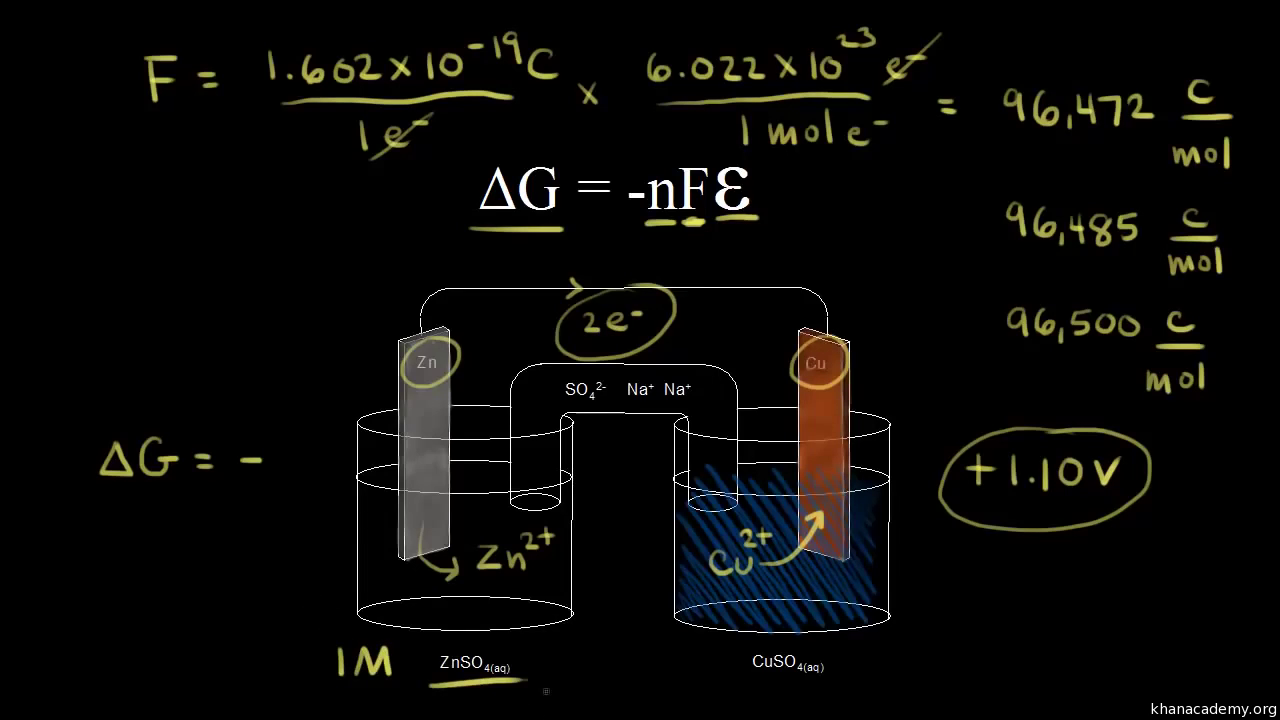
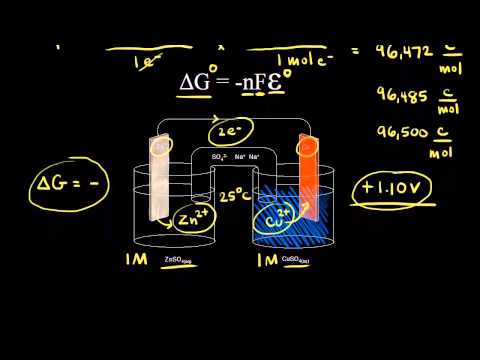
Merging electrochemistry with thermodynamics gives this formula. This relationship is as follows. And were going to calculate the value for delta g at the end of this video.
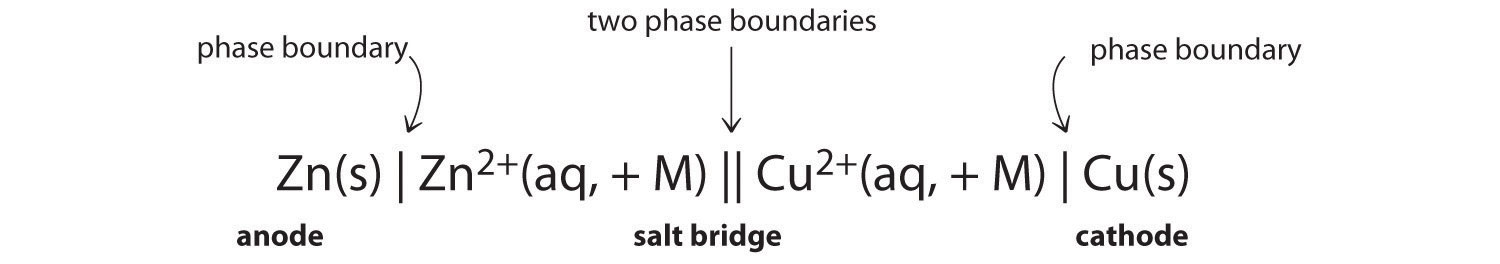
In chemistry a reaction quotient is a function of the activities or concentrations of the chemical species involved in a. Consequently there must be a relationship between the potential of an electrochemical cell and deltag. N is the moles of electrons moved around.
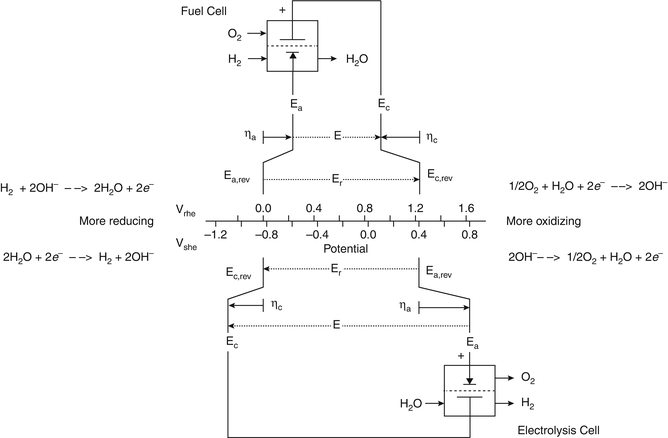
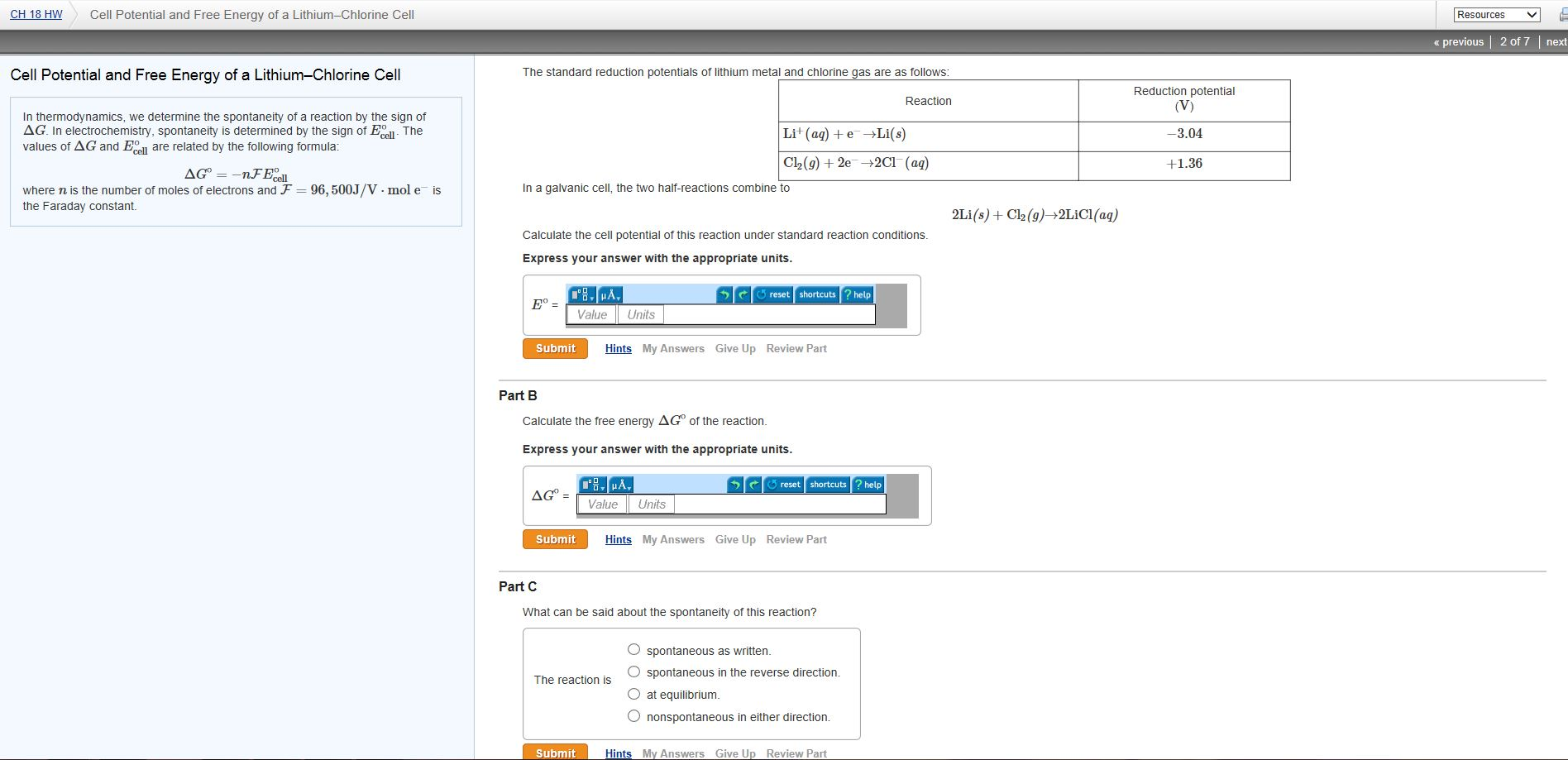
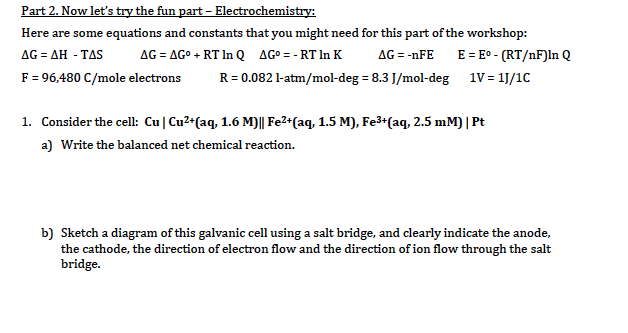
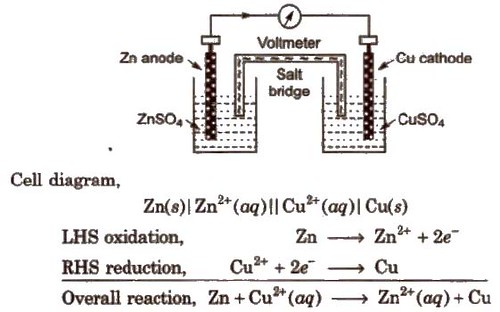
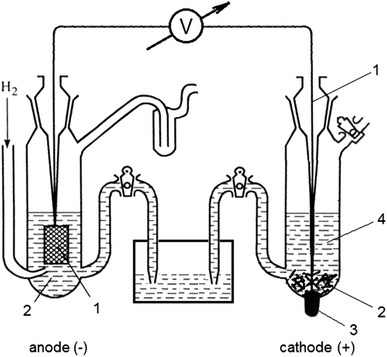
Chemistry stack exchange is a question and answer site for scientists academics teachers and students in the field of chemistry. When you acquire this put it into the formula delta g0 nfe0 where f is the constant 96485 coulumbs per mole of electrons. It can be applied to a cell though so if the hydrogen electrode is connected to another electrode say copper dipped in copper sulfate solution then you can find the free energy.
To check it use the formula. Delta g n f ecell cell potential is different for each voltaic cell. Its value depends upon the concentrations of specific reactants and products as well as temperature of the reaction.
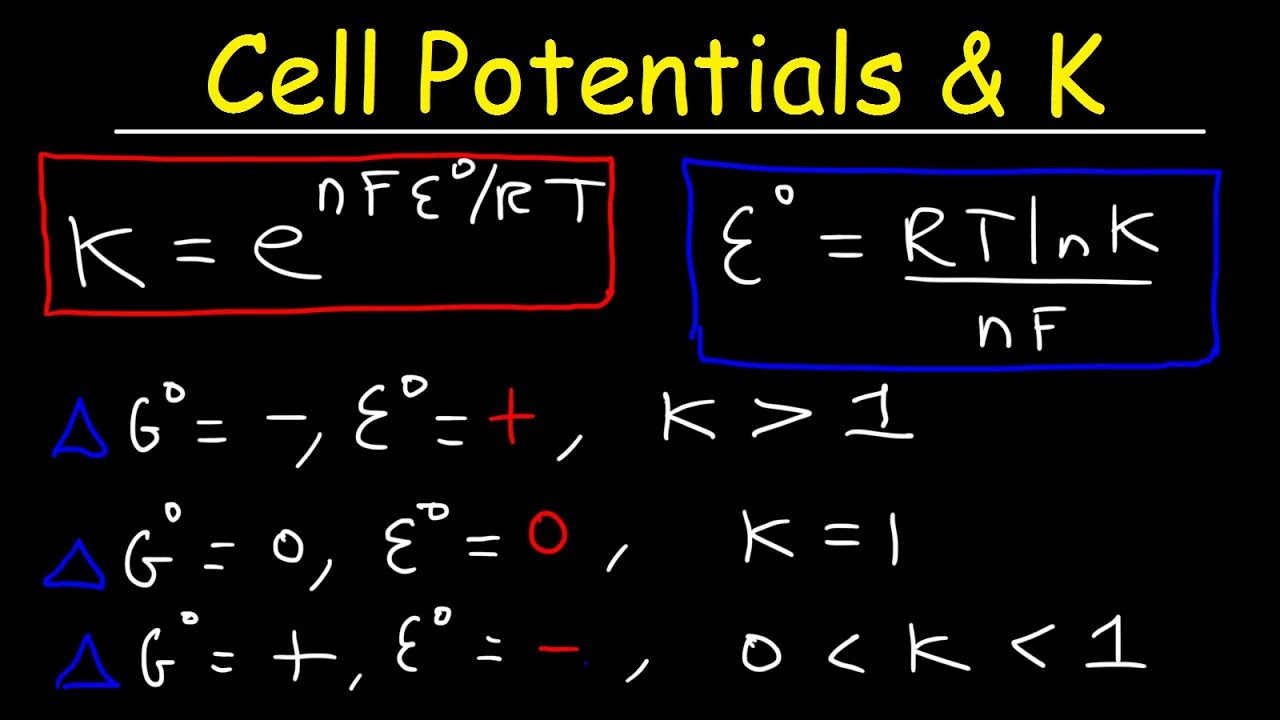
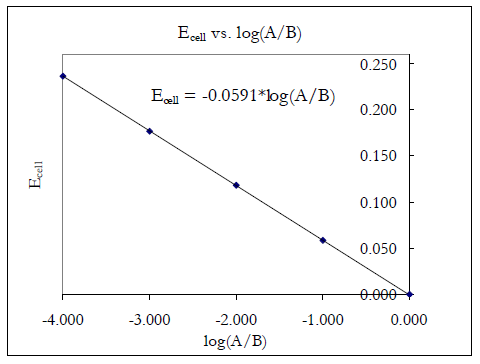
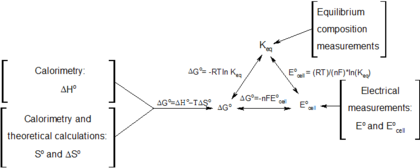
Log k ne00591 but only if at stp. If we know the standard state free energy change g o for a chemical process we can calculate the cell potential e o for an electrochemical cell based on that process using the relationship between g o and e o. Its better to remember the formula as.
It only takes a minute to sign up. So delta g delta g is the change in free energy. Now you have delta g.
The change in free energy deltag is also a measure of the maximum amount of work that can be performed during a chemical process dg wmax.