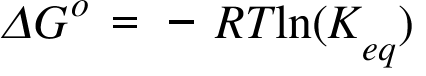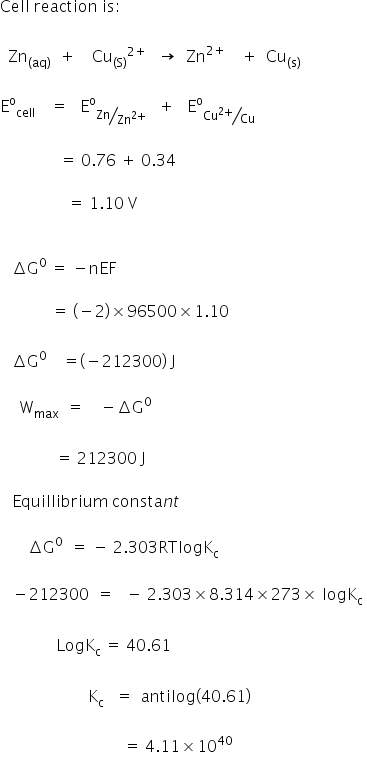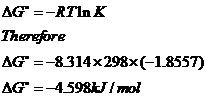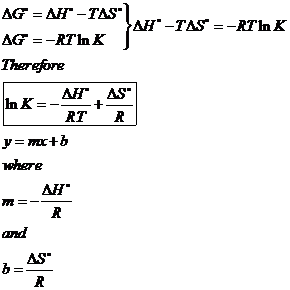Delta G Degree Formula

The Relationship Between Free Energy And The Equilibrium Constant Video Lesson Transcript Study Com
study.com
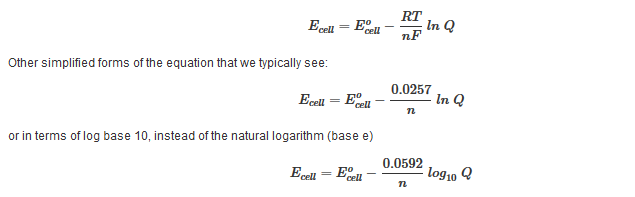
17 4 The Nernst Equation Chemistry 112 Chapters 12 17 Of Openstax General Chemistry
psu.pb.unizin.org
This statement as well as the degree sum formula is known as the handshaking lemmathe latter name comes from a popular mathematical problem to prove that in any group of people the number of people who have shaken.

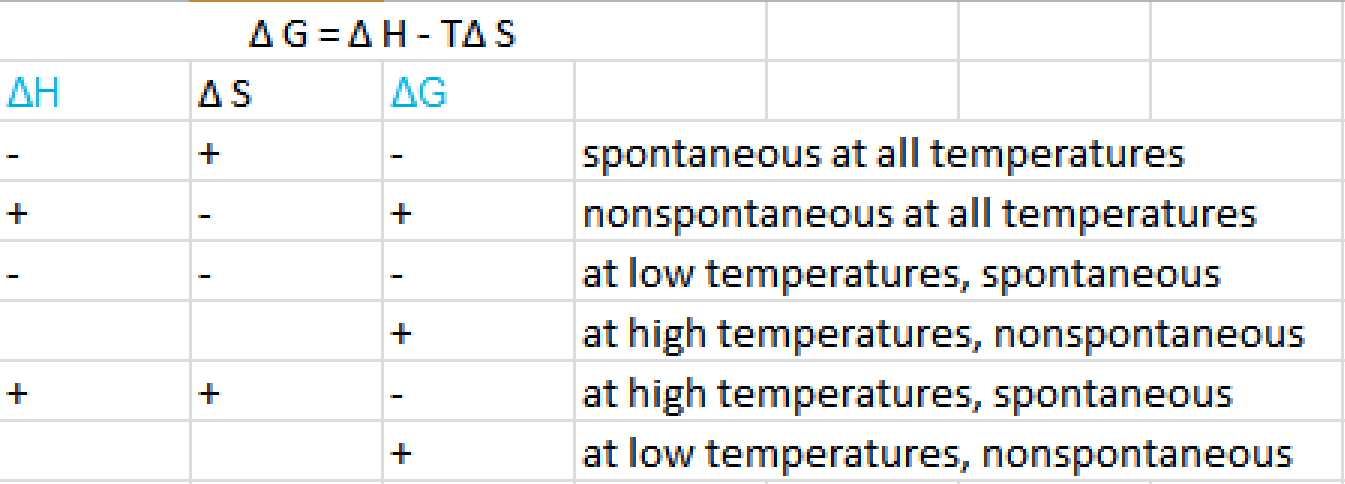
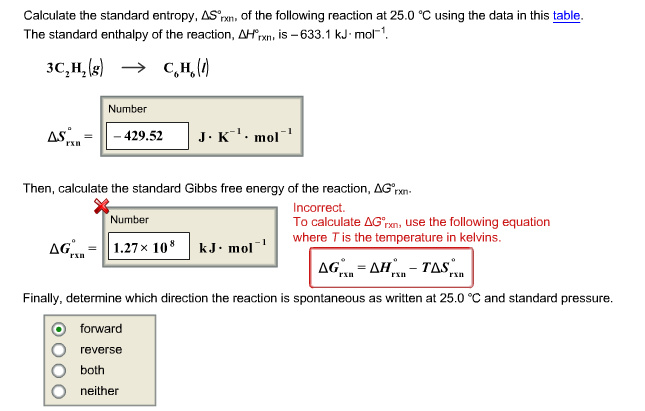
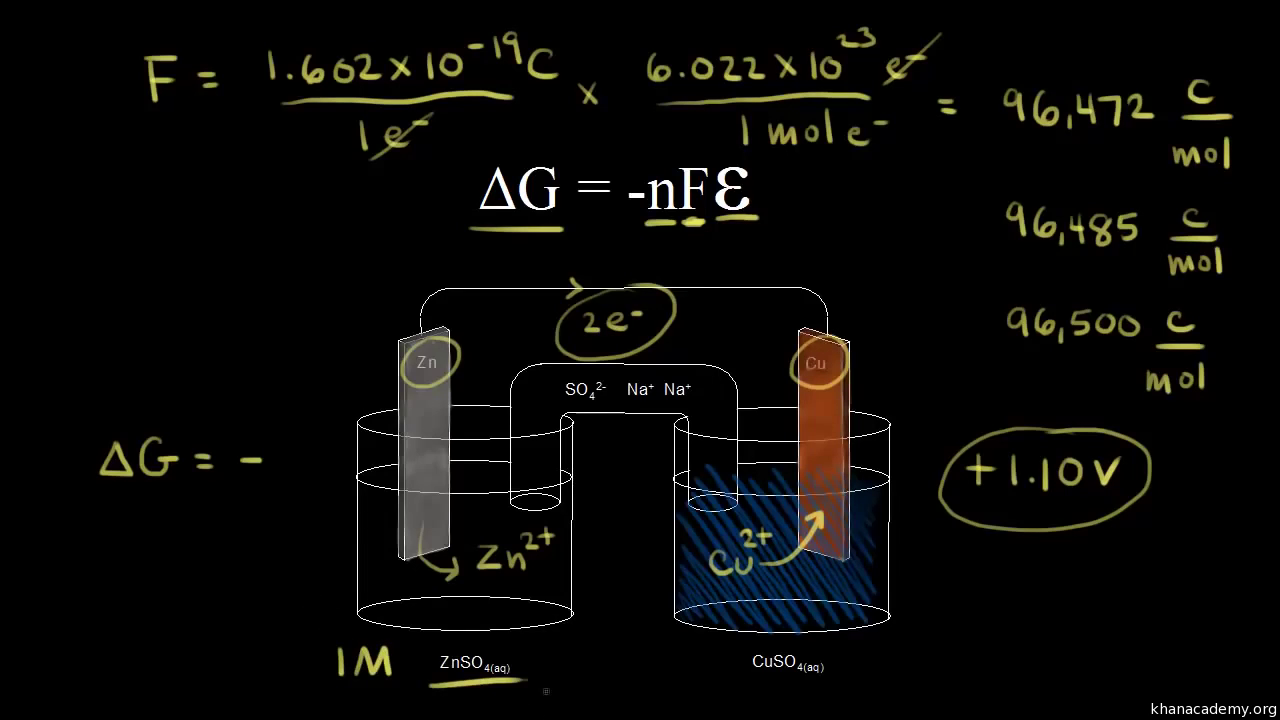
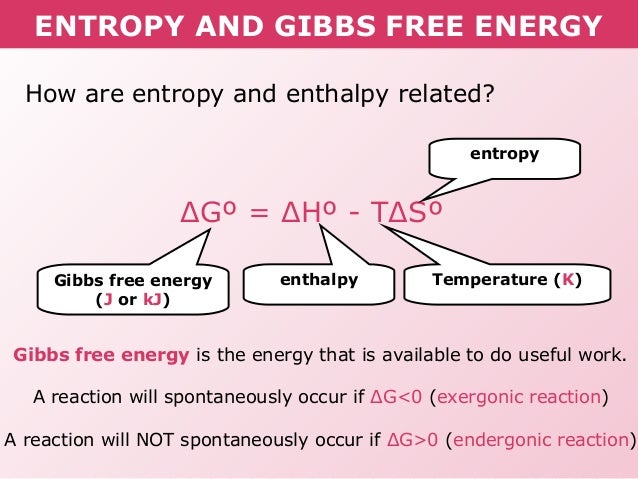
Delta g degree formula. However again because all of these are linked to chemistry and because chemistry likes to measure everything per mole all of the variables above but temperature may also have attached to them per mole mol. As such i think that knowledge of it and the consequences associated with it are. T is in the units of kelvin k.
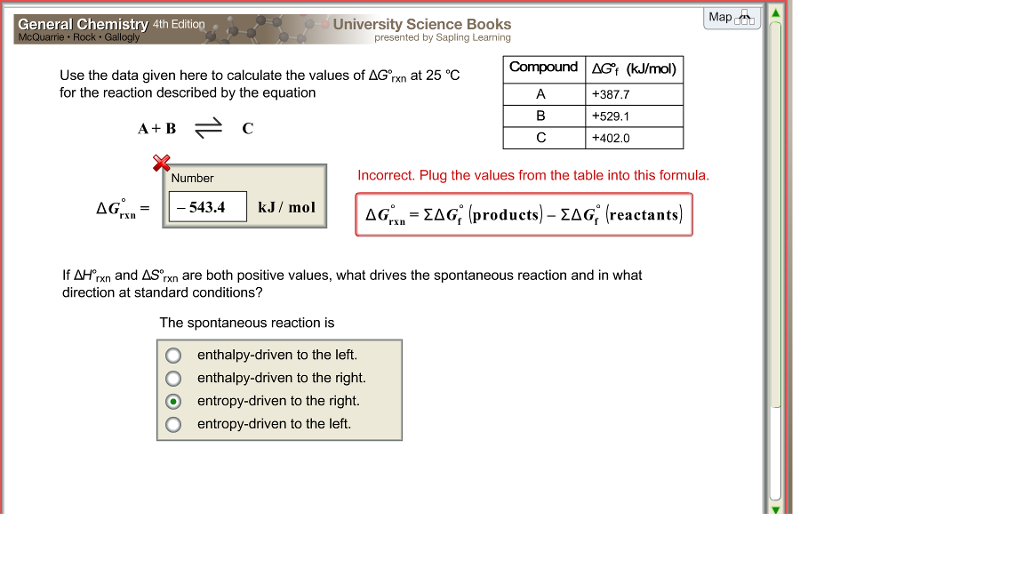
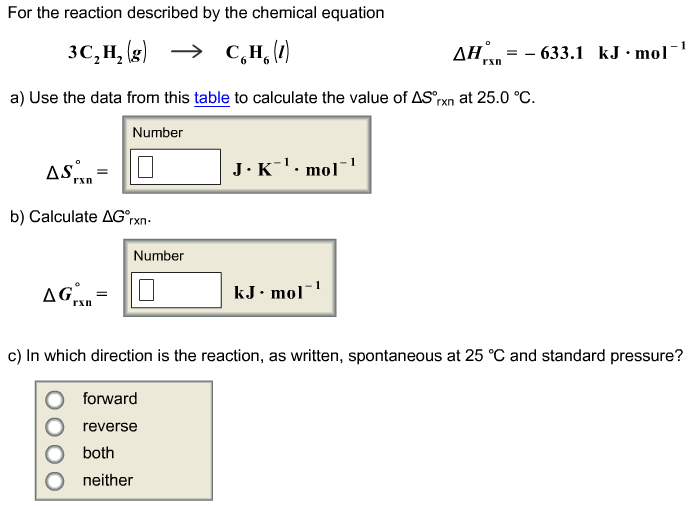
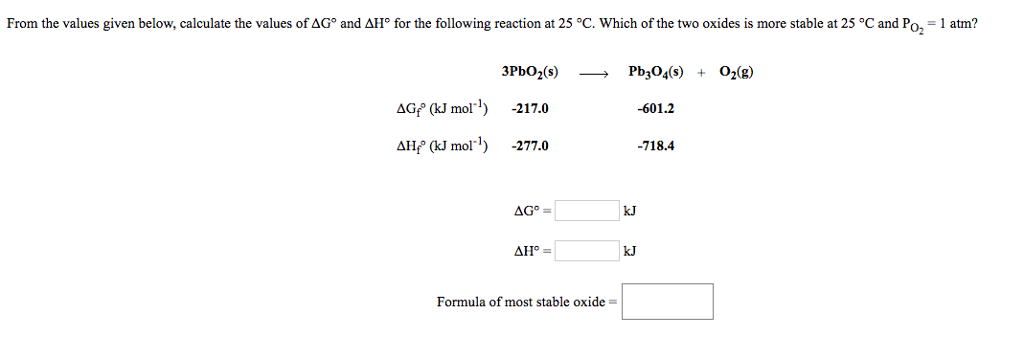
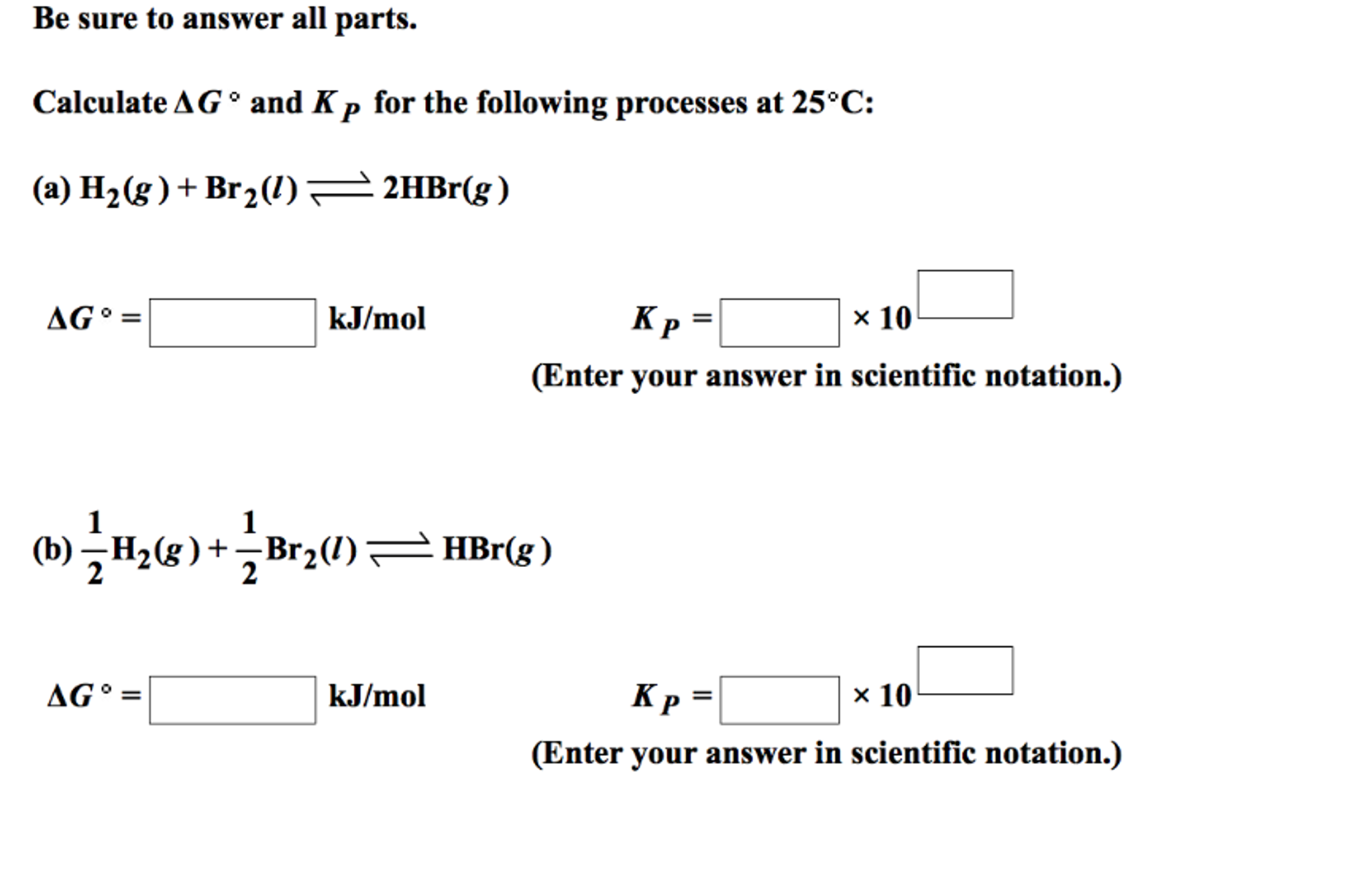
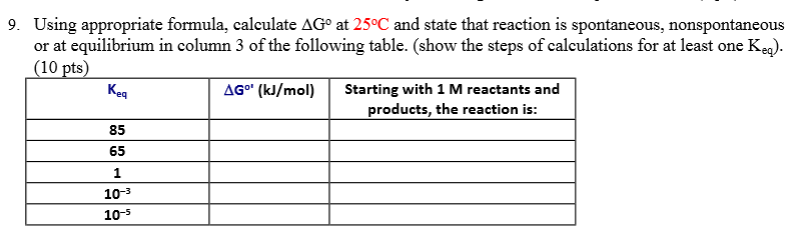
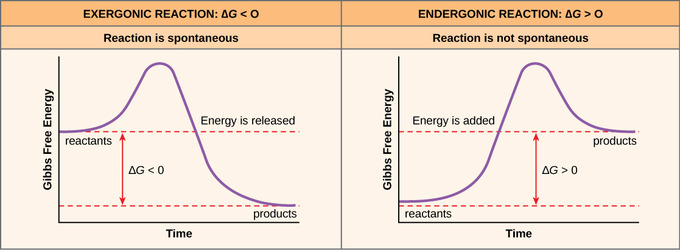
G o for a reaction can be calculated from tabulated standard state free energy data. I thought maybe theyre just asking for the standard g so i used the values from the table and subtracted the products from the reactants. I tried this formula.
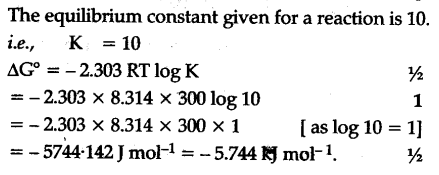
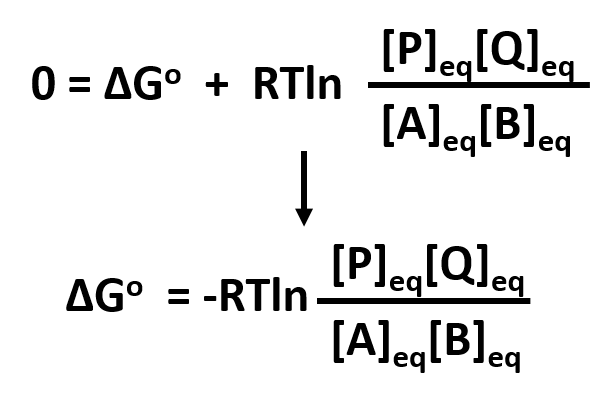
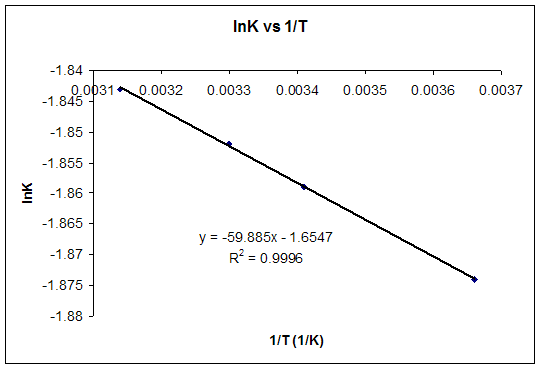
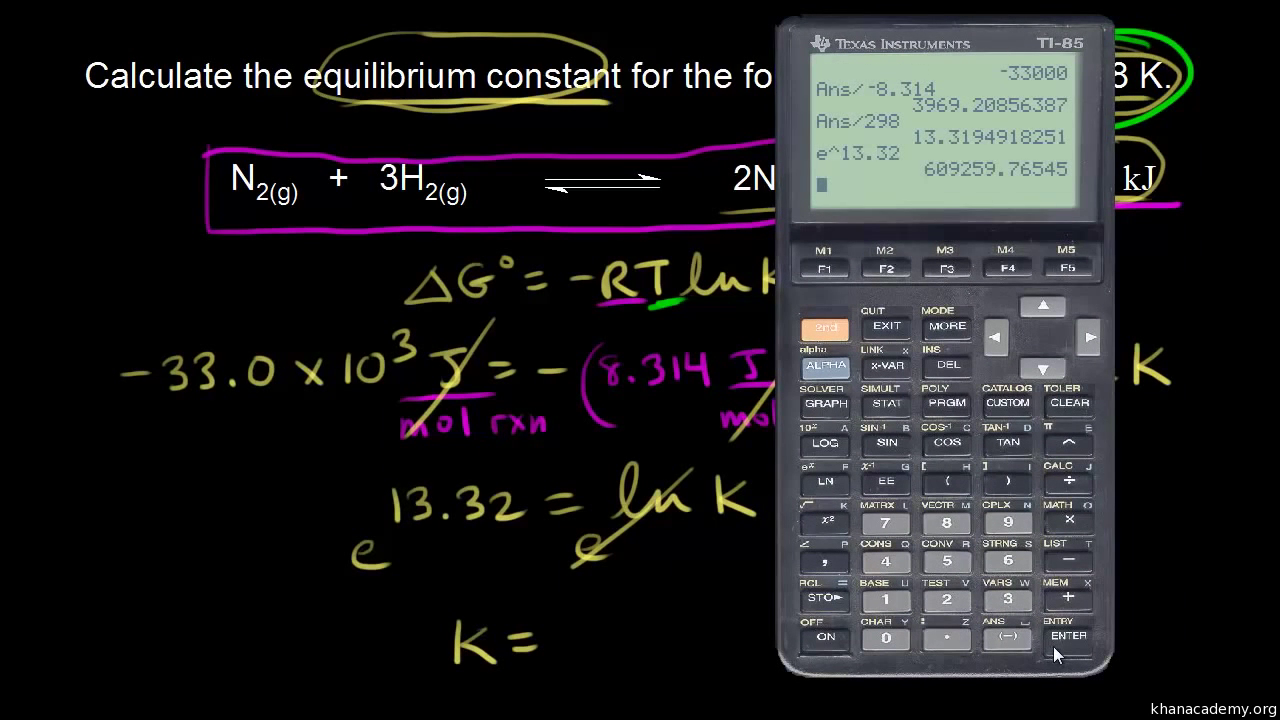
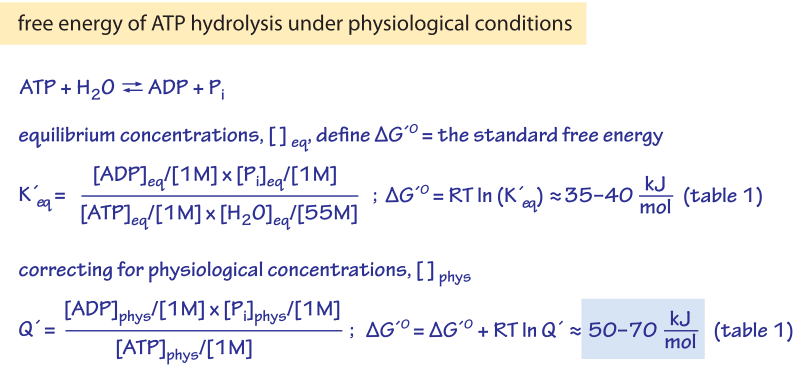
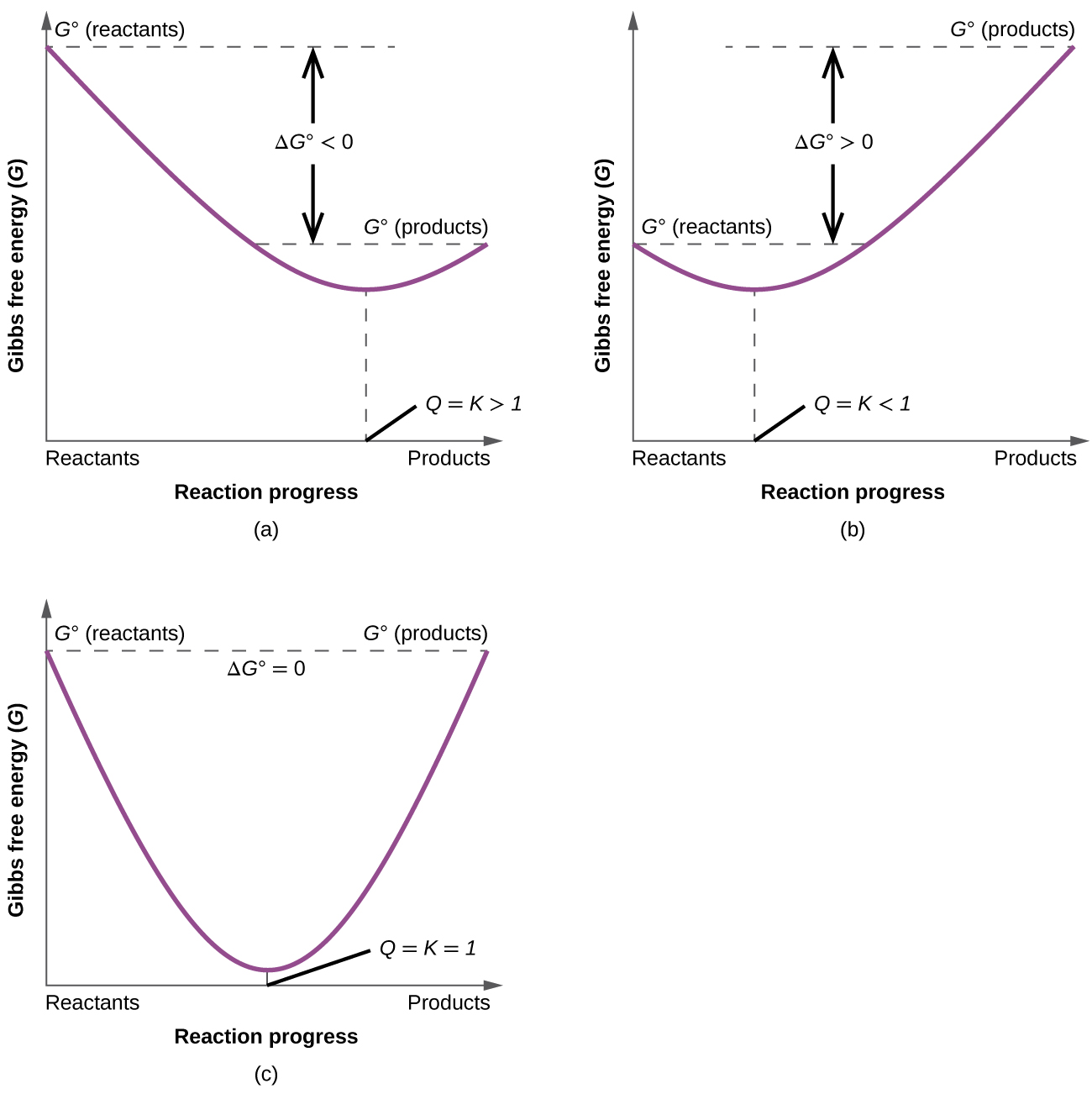
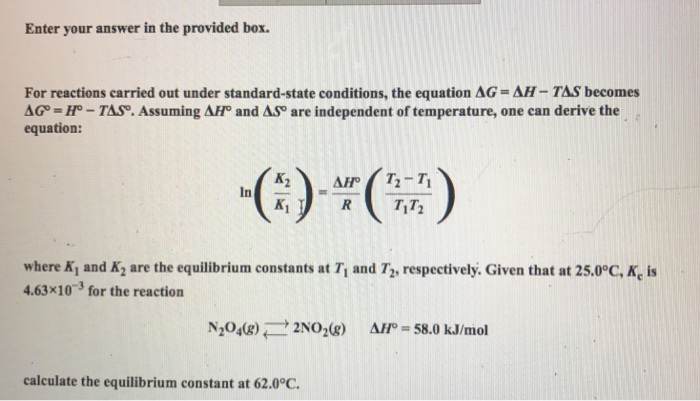
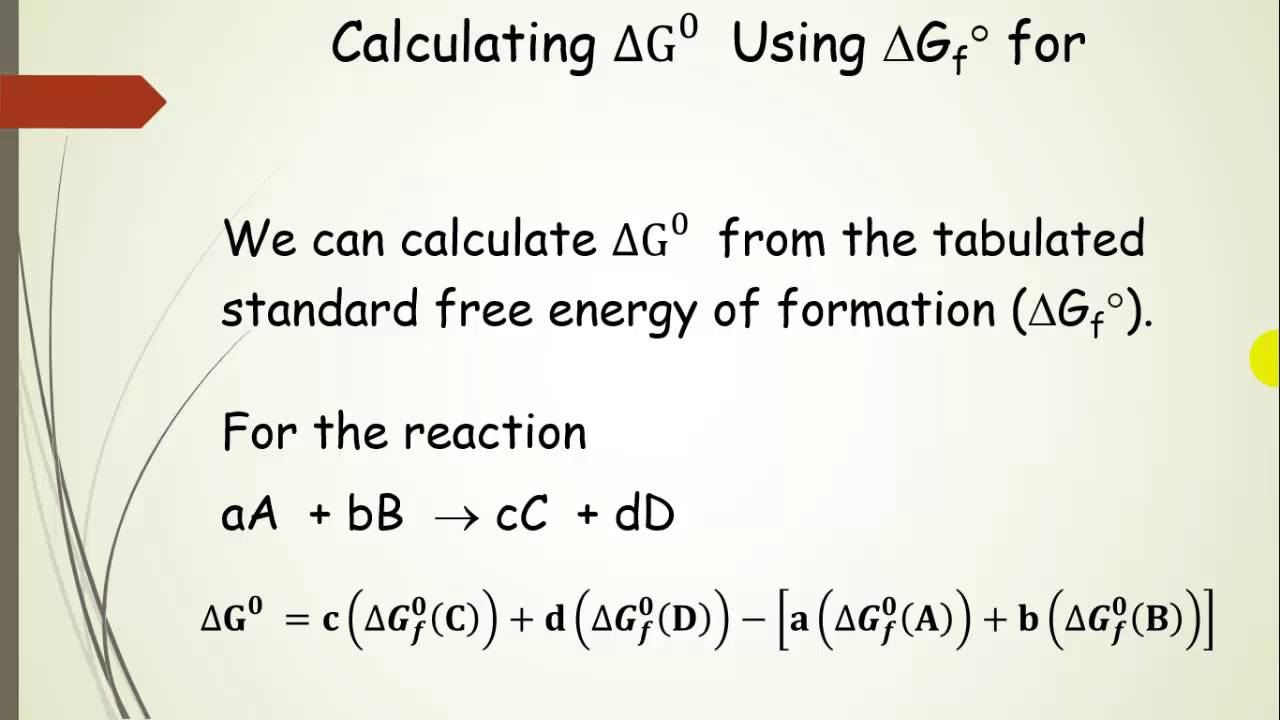
If we know the standard state free energy change g o for a chemical process at some temperature t we can calculate the equilibrium constant for the process at that temperature using the relationship between g o and k. This is the free energy change for a reaction that is not at the standard state. As might be expected the standard state free energy of formation of a substance is the difference.
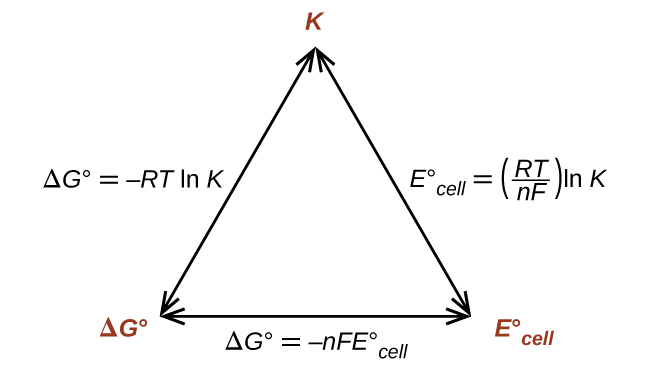
Cog cl2 g cocl2g and the kp 562 x 1035. If we know the standard state free energy change g o for a chemical process we can calculate the cell potential e o for an electrochemical cell based on that process using the relationship between g o and e o. Consider the two equations that deal with delta g g.
T is the temperature on the kelvin. R 8314 j mol 1 k 1 or 0008314 kj mol 1 k 1. One of the changes was to remove equation 2 below from the equations constants sheet.
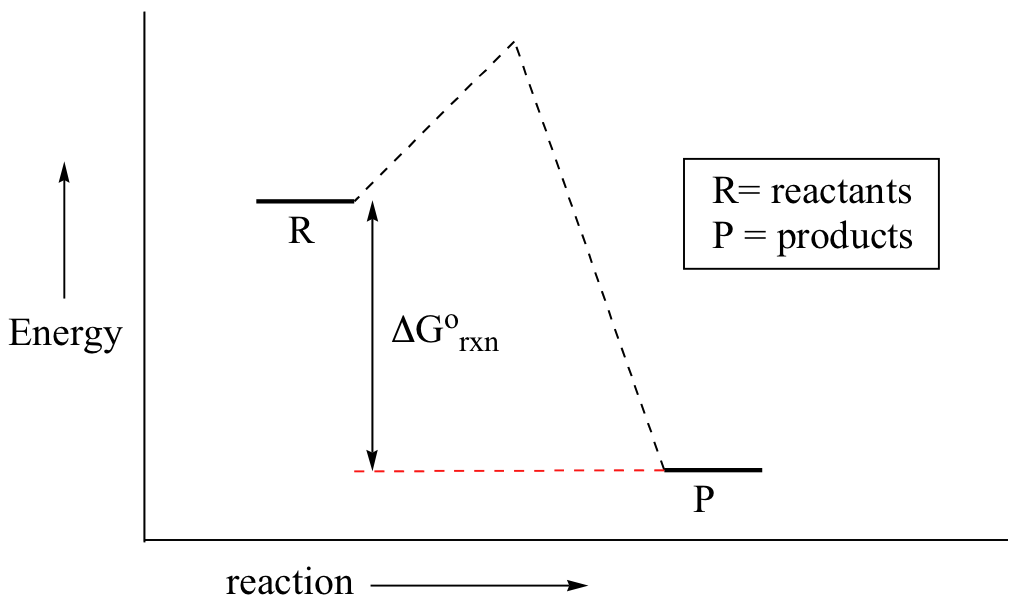
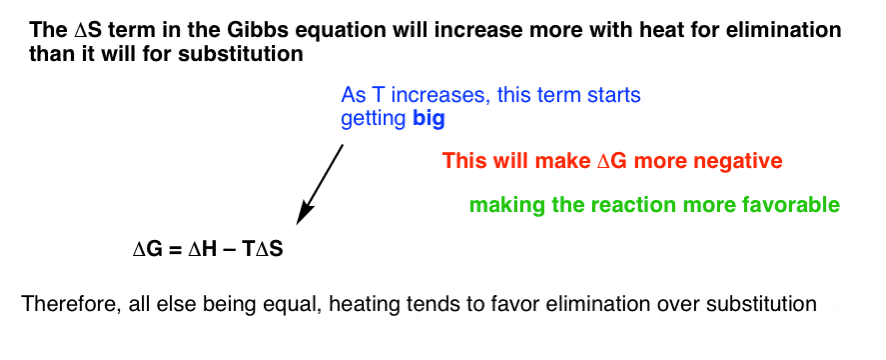
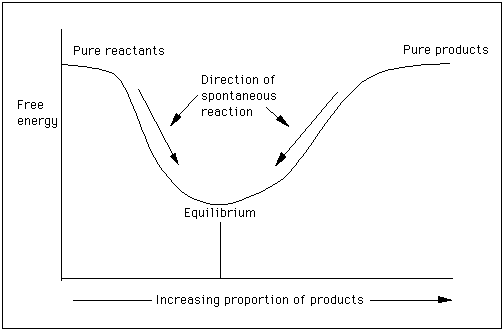
According to the second law of thermodynamics for systems reacting at standard conditions for temperature and pressure or any other fixed temperature and pressure there is a general natural tendency to achieve a minimum of the gibbs free energy. Rearrangement gives in this equation. Since this post was originally written in january 2012 the ap exam has changed.
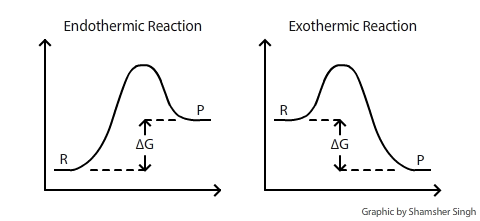
D s is in the units of joules per kelvin j k. D h is in the units of joules j. A quantitative measure of the favorability of a given reaction at constant temperature and pressure is the change dg sometimes written delta g.
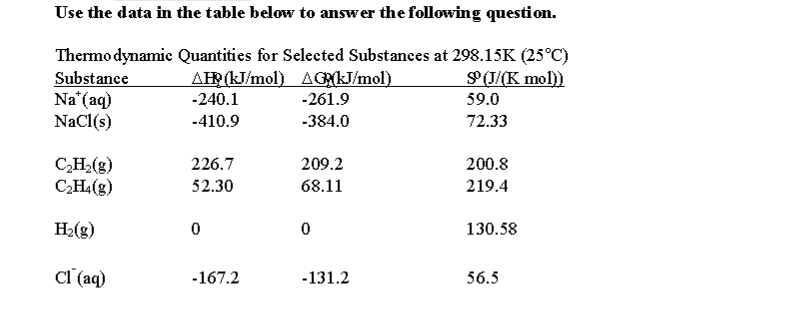
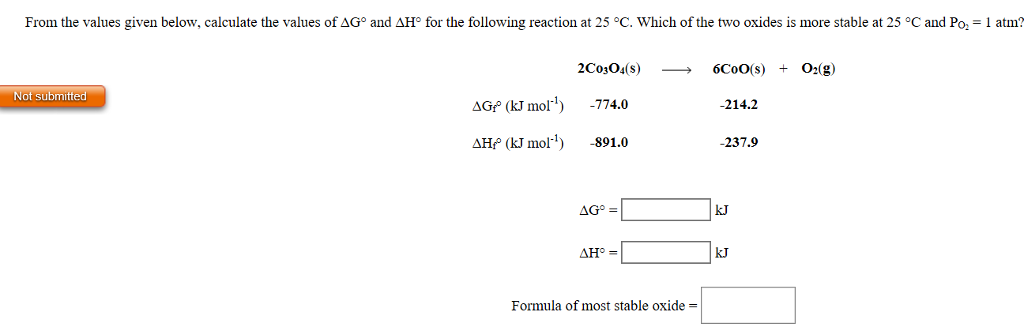
D g o a delta g with a superscript o is the free energy change for a reaction with everything in the standard states gases at 1 bar and solutions at 1 m concentration and at a specific temperature usually 250c d g just delta g. Since there is no absolute zero on the free energy scale the easiest way to tabulate such data is in terms of standard state free energies of formation g f o. D g is in the units joules j.
The degree sum formula states that given a graph.
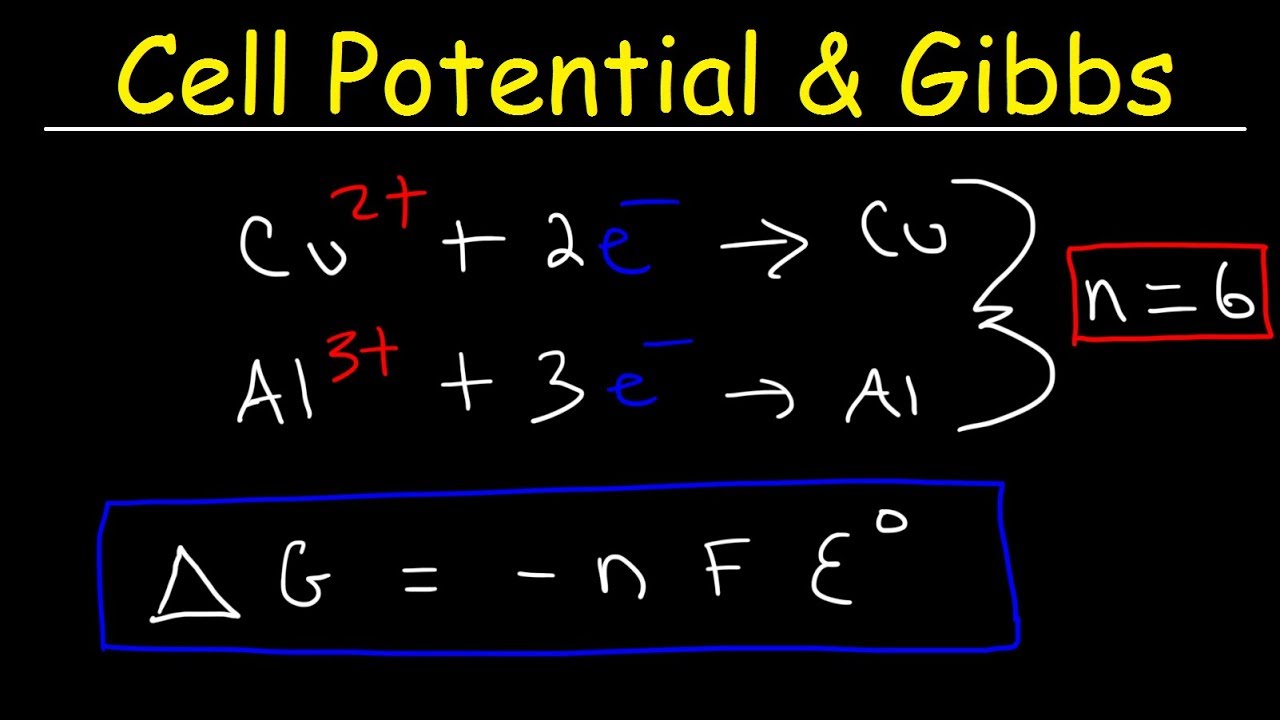
Cell Potential Gibbs Free Energy Standard Reduction Potentials Electrochemistry Problems Youtube
www.youtube.com