Nmm Chemistry Formula
N in chemistry stands for.
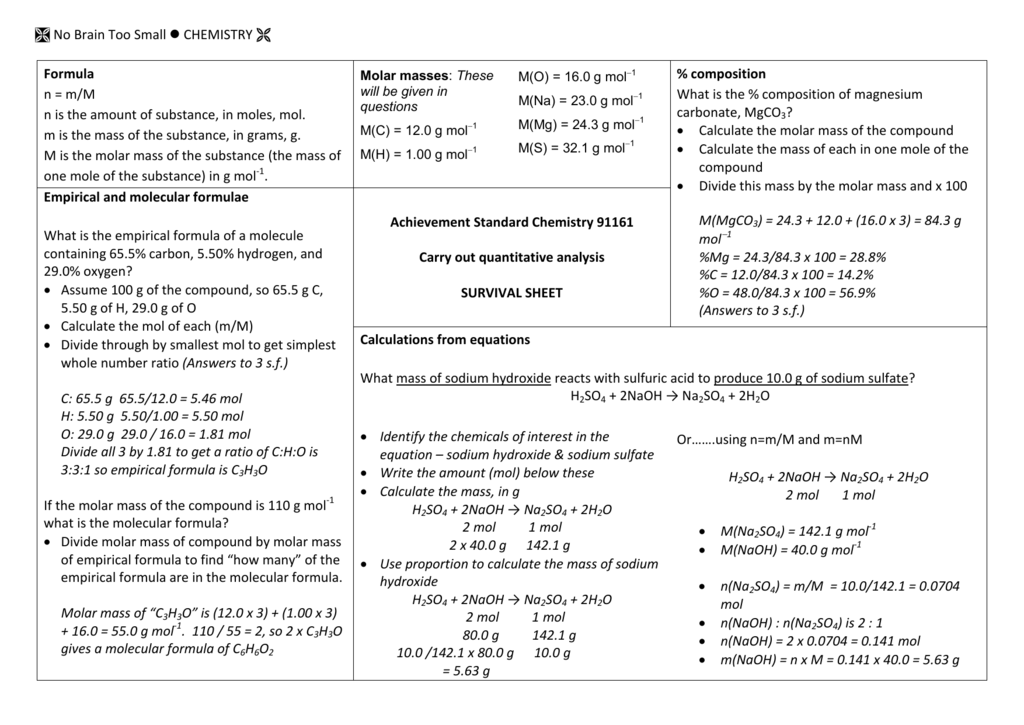
Nmm chemistry formula. Chemical formulae provide insight into the elements that constitute the molecules of a compound and also the ratio in which the atoms of these elements combine to form such molecules. Mivi mfvf boyles law constant t and n. A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule using chemical element symbols numbers and sometimes also other symbols such as parentheses dashes brackets commas and plus and minus signs.
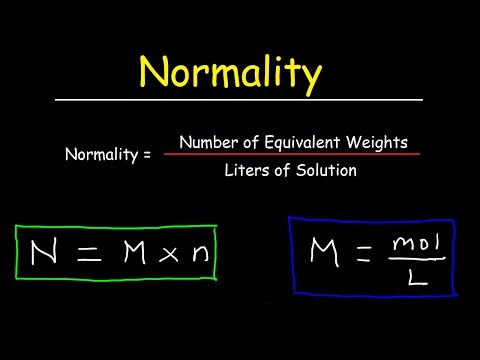
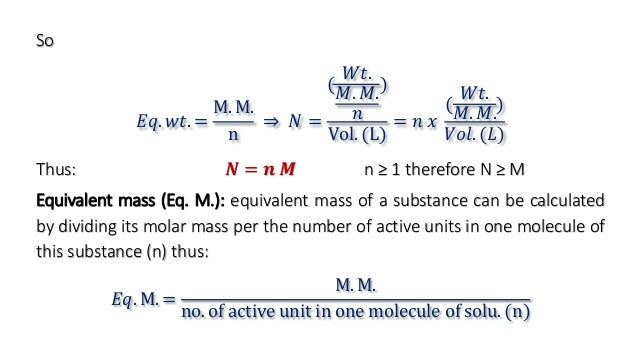
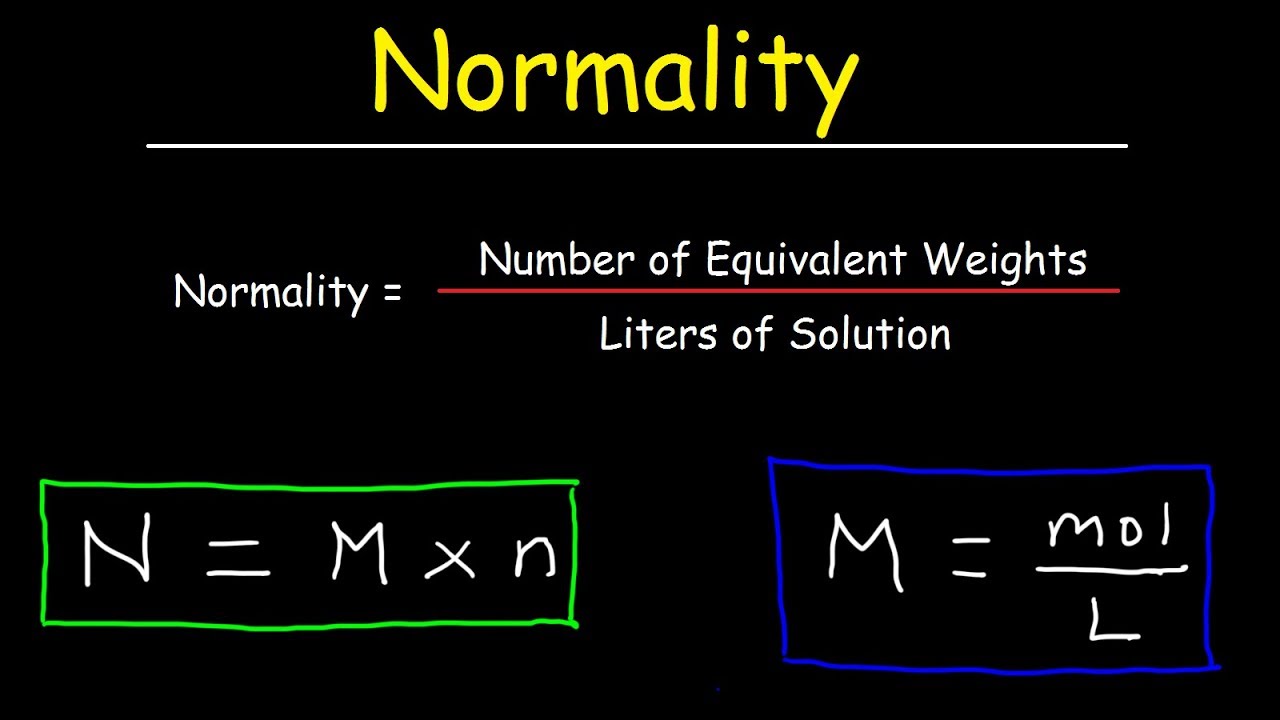
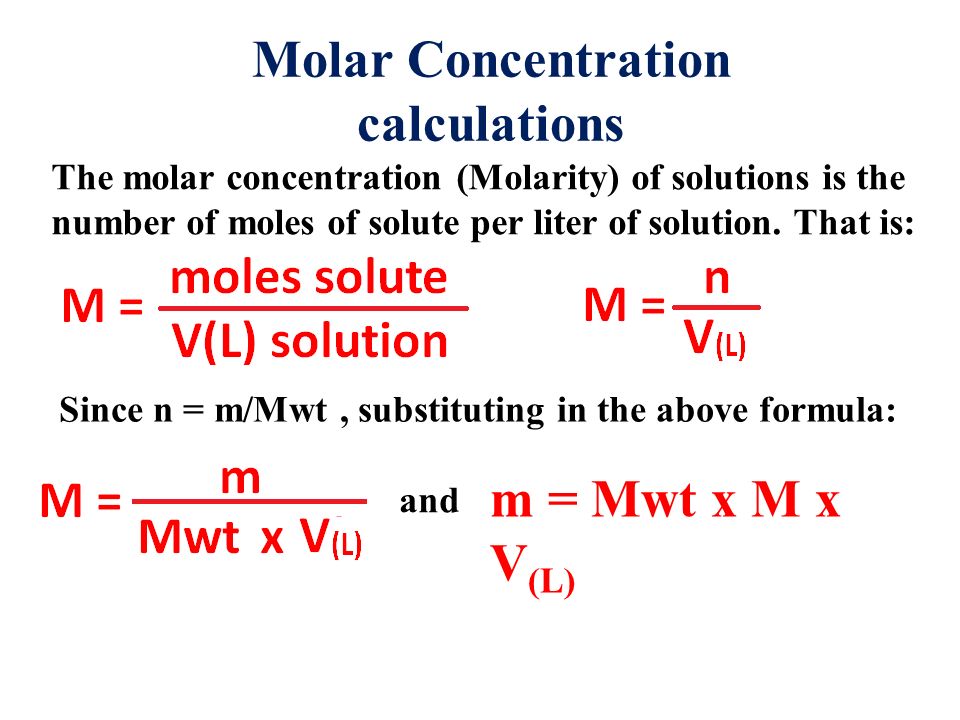
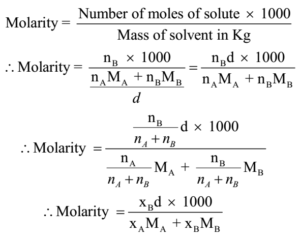
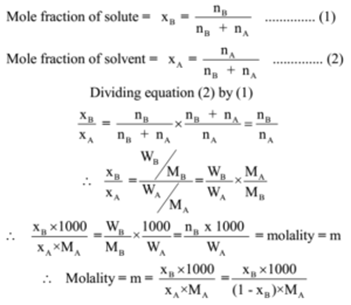
Extract the data from the question. M is the molar mass of the substance the mass of one mole of the substance in g mol 1. A solution using these units is called a molal solution eg 01 m naoh is a 01 molal solution of sodium hydroxide.
Using these formulas the 4 questions are simple. M is the mass of the substance in grams g. Both m and m are units of the concentration of a chemical solution.
Mthese will be given in questions. A certain room has a volume of 100 m3 and we know that there are 4 groups of people for every m3. So how many groups of people are in the room.
The lowercase m indicates molality which is calculated using moles of solute per kilograms of solvent. Chemical elements periodic table compound name formula search moles to grams calculator common compounds list chemical equation balancer complete list of acids complete list of bases molar to mass concentration converter molar mass calculator cations anions list dilution calculator molarity calculator. What does n stand for in the formula nmm.
The second one is nmmr where n is the number of moles again m is the mass in grams and mr is the molar mass atomic mass of each element and can be worked out easily given the chemical. N175mr of ch4124. N is the amount of substance in moles mol.
When calculating mass of a formula you use this formula i have had a mingd black and cannot remember what n means can anyone help me as i am studying for exams. N mm. M n m substitute the values into the equation and solve for mass g.
N mol c molm3 v m3 note that m3 might be l cc cm3 ml in3 or any other measure of volume. No brain too small chemistry formula. 1 first we want to find out how many moles of ch4 we have so we use nmmr.
These are limited to a single typographic line of symbols which may include. What m and m mean in chemistry. For example the chemical formula of water which is h2o suggests.
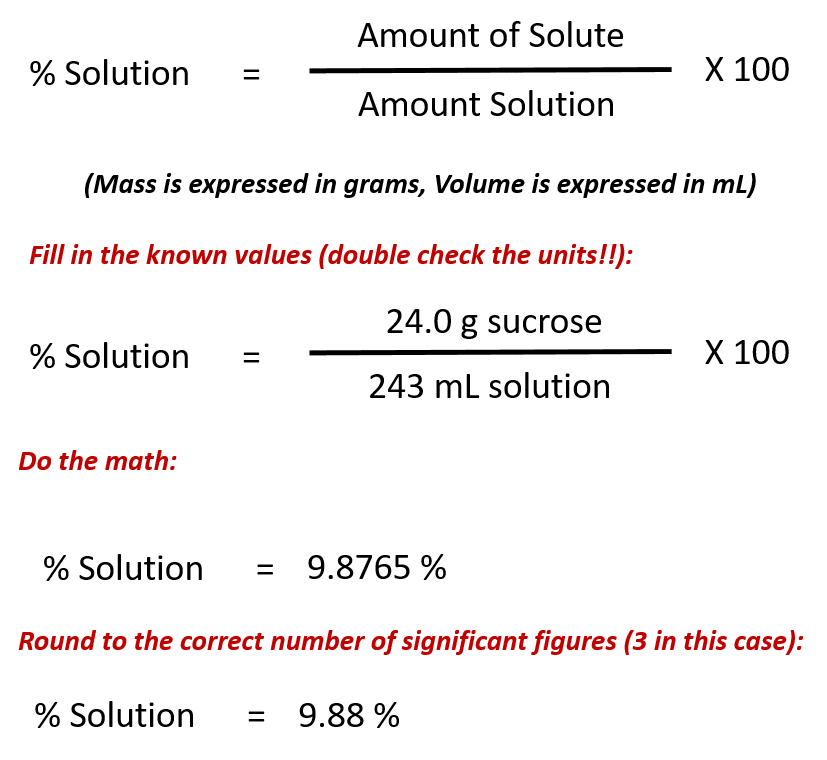
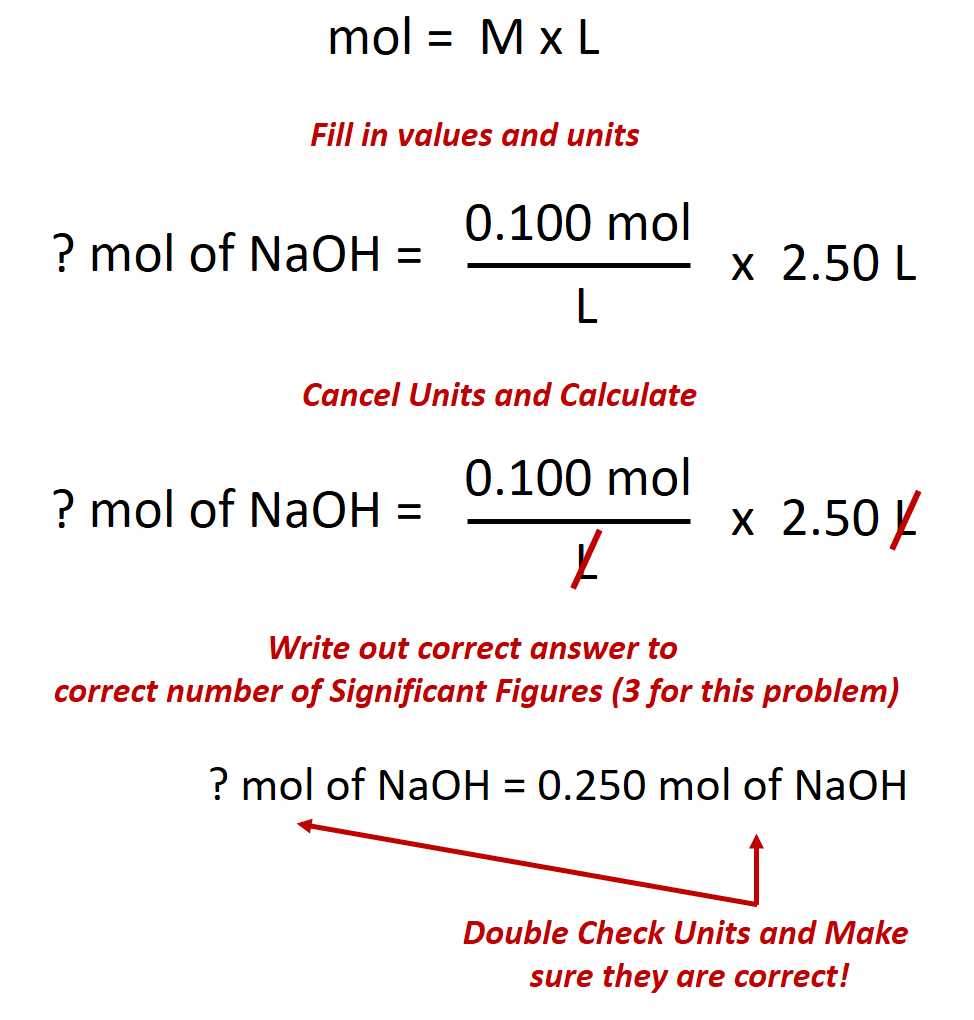
100m3 4 groupsm3 400 groups. Mass m 1245 g. N x molar mass of element molar mass of compound x 100 where n the number of moles of the element in one mole of the compound yield actual yield theoretical yield x 100 molarity m moles of solute liters of solution dilution of solution.
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gctctrub3z Bcv Ahp Euuqsreedscgrdxvmuqia5g7w Lirykae Usqp Cau
encrypted-tbn0.gstatic.com

Pdf Synthesis Crystal Structure Mesophase Behaviour And Optical Property Of Azo Ester Bridged Compounds
www.researchgate.net

Using The Ideal Gas Law To Calculate A Change In Volume Worked Example Video Khan Academy
www.khanacademy.org












































/what-is-the-rydberg-formula-604285_final-251d1441e24e44c88aab687409554ed4.png)






/606823-calculate-molarity-of-a-solution-FINAL-5b7d7e15c9e77c0050355d4e.png)



/how-to-calculate-normality-609580final2-0d5efa5a961f4fa0a7efc780921faee1.png)