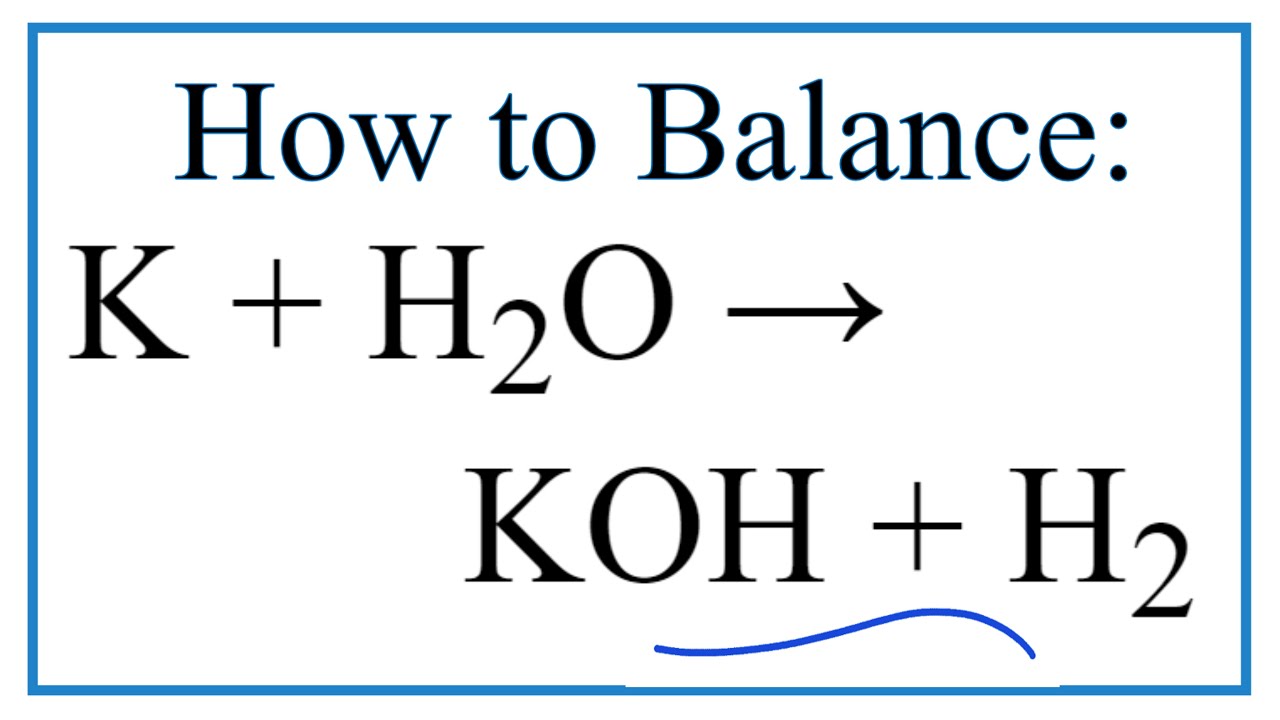
K Formula Chemistry
Changes in reaction conditions can have a tremendous effect on the course of a redox reaction.

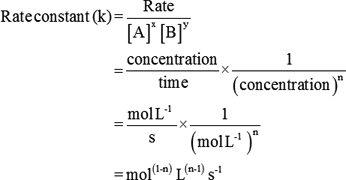
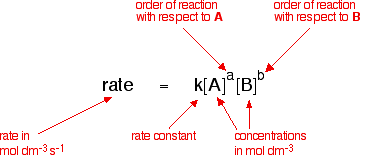
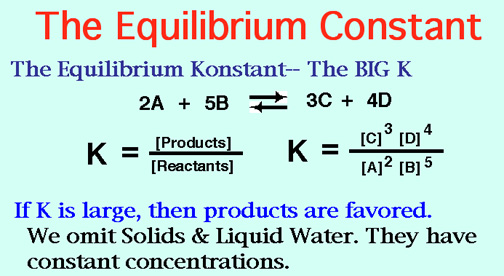
K formula chemistry. Use correct case for element symbols. This state of equilibrium can be described by the equilibrium constant k. For example under standard conditions the reaction of cos with ni 2 aq to form nis and co 2 aq occurs spontaneously but if we reduce the concentration of ni 2 by a factor of 100 so that ni 2 is 001 m then the reverse reaction occurs spontaneously instead.
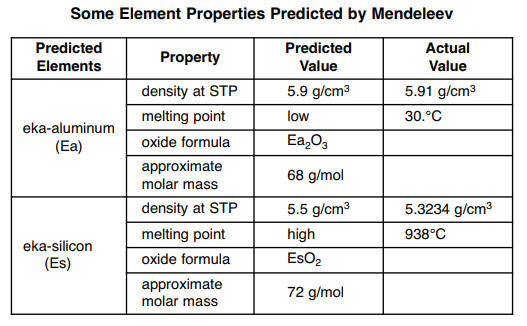
To score good marks in chemistry students have to memorize the chemical formula by heart. Chemical formulae provide insight into the elements that constitute the molecules of a compound and also the ratio in which the atoms of these elements combine to form such molecules. This page explains what is meant by an equilibrium constant introducing equilibrium constants expressed in terms of concentrations k cit assumes that you are familiar with the concept of a dynamic equilibrium and know what is meant by the terms homogeneous and heterogeneous as applied to chemical reactions.
The value of k w is constant at particular temperature. At room temperature the value of equilibrium constant k w is 100 x 10 14 mol 2 dm 6. If correct case is not used the formula may be ambiguous and the interpretation chosen may not be the desired one.
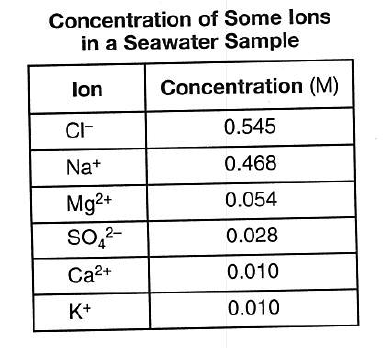
Though these formulas are found to be very hard to learn and understand very necessary to solve the reaction. Chemical formula search help rules for chemical formulas back to search enter a sequence of element symbols followed by numbers to specify the amounts of desired elements eg c6h6. For example the chemical formula of water which is h2o suggests.
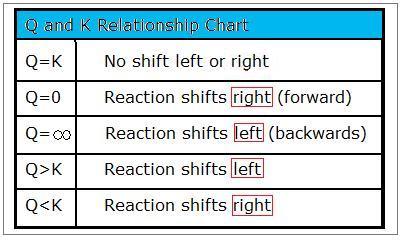
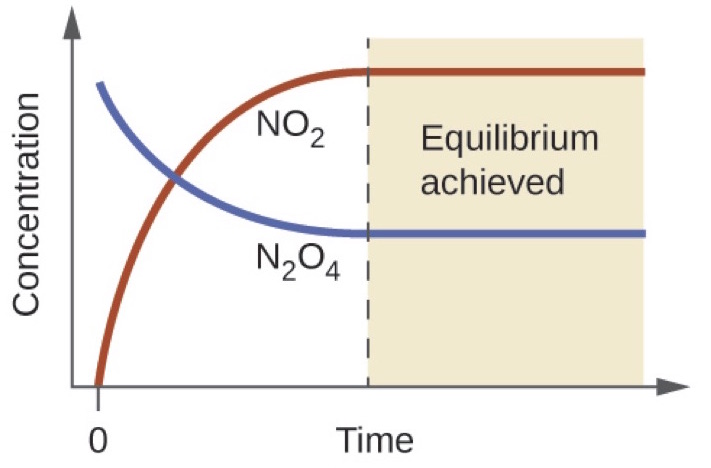
Autoionisation of water is an endothermic process. This is when the forward and reverse reactions occur at equal rates. The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency towards further changefor a given set of reaction conditions the equilibrium constant is independent of the initial analytical concentrations of the.
Chemical equilibrium is the state in which the reactants and products have no net change over time. Chemical formulas can be quite simple sometimes as h hydrogen or it can take a rather complicated form such as ch 3 ch 2 oh ethanol. Value of k w.
K w increases with increase of temperature.

When Do Reactions Reach Chemical Equilibrium And Why Does Chemical Equilibrium Occur
masterconceptsinchemistry.com

Amazon Com Laminated Color Periodic Table And Formula Sheet For Chemistry Biochemistry And Physics Home Audio Theater
www.amazon.com











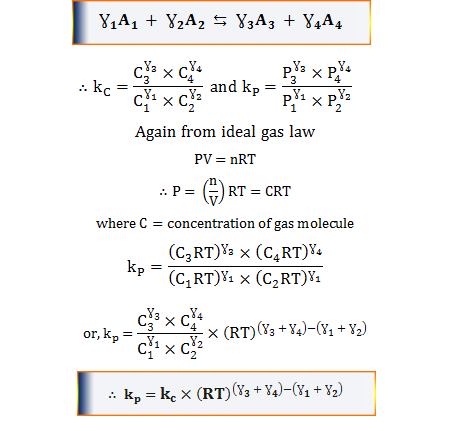



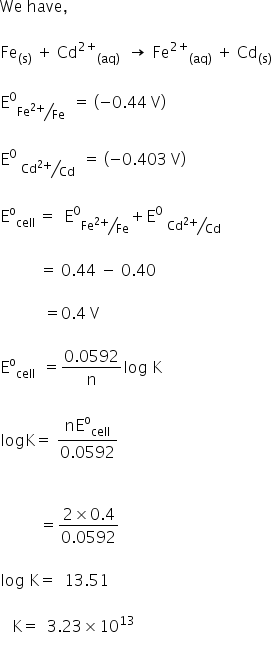












/ThoughtCo_Convert_Fahrenheit_To_Kelvin_609231_V1-5ca840ca9d8f4e90be33ffb0fa72a182.gif)




















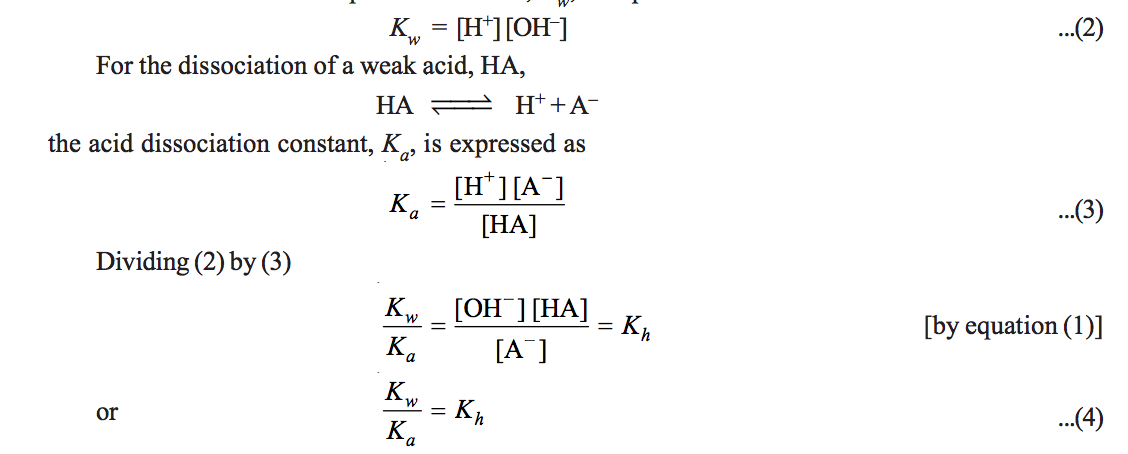













/ThoughtCo_Convert_Kelvin_To_Fahrenheit_609234_V2-e1495e4d48814c63a03b96ea13c45d43.gif)


