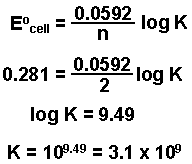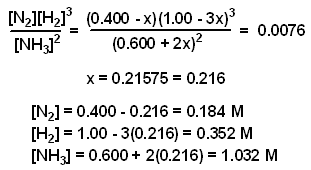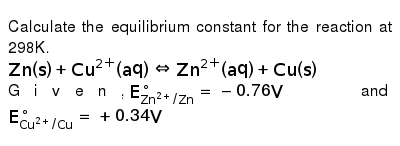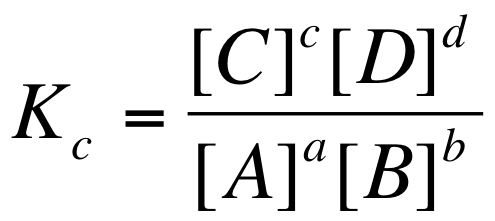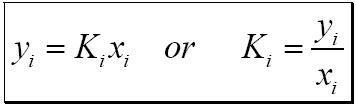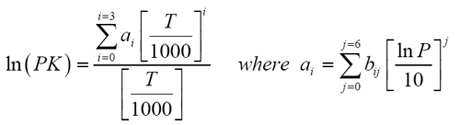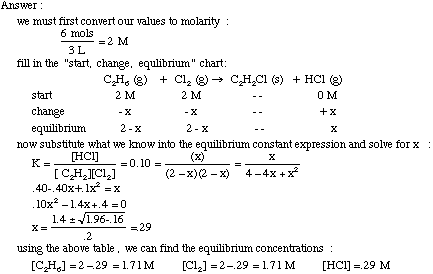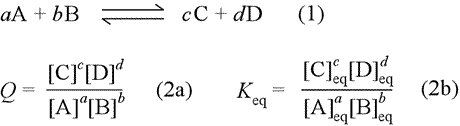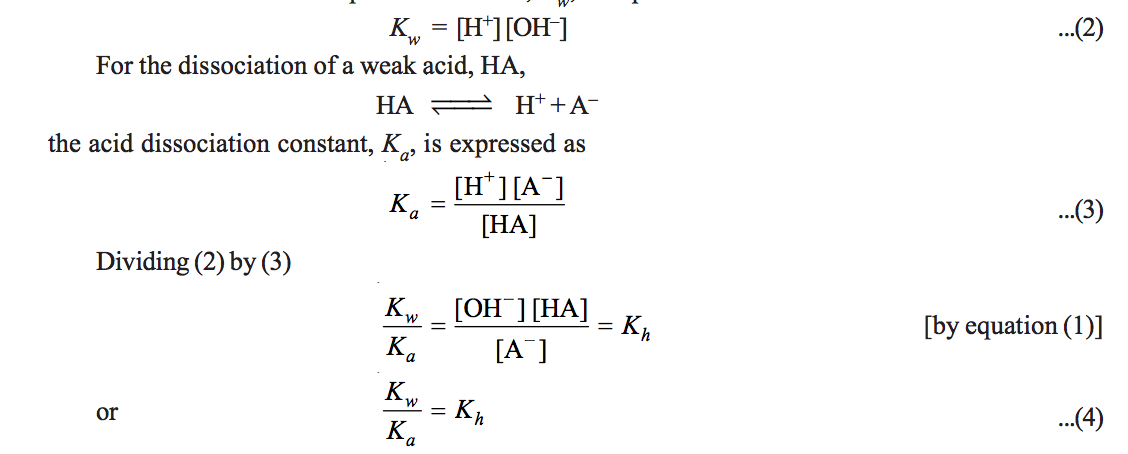K Equilibrium Formula
This page explains equilibrium constants expressed in terms of partial pressures of gases k pit covers an explanation of the terms mole fraction and partial pressure and looks at k p for both homogeneous and heterogeneous reactions involving gases.
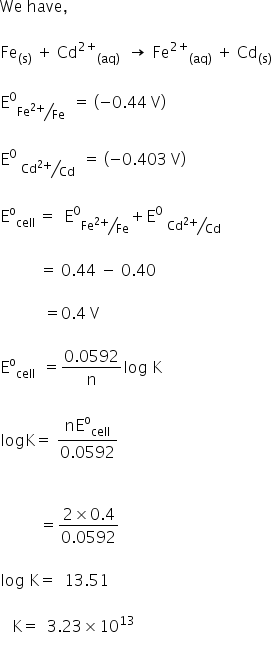
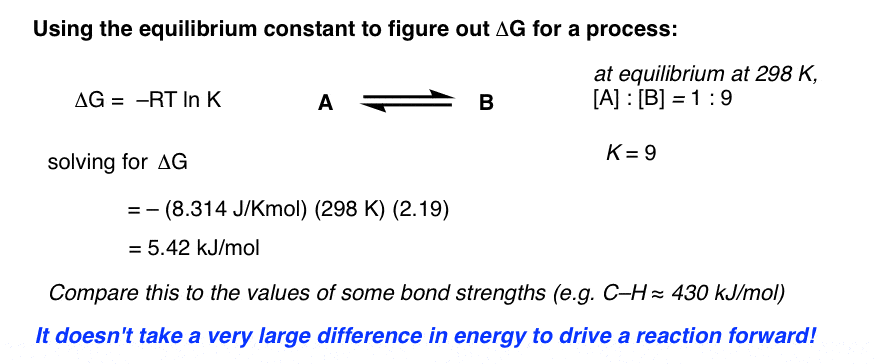
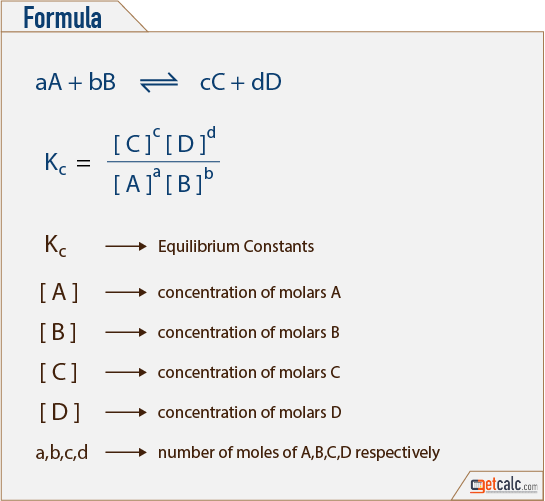
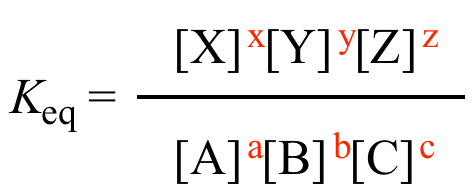
K equilibrium formula. The equilibrium constant is the value of the reaction quotient that is calculated from the expression for chemical equilibriumit depends on the ionic strength and temperature and is independent of the concentrations of reactants and products in a solution. The contact process equilibrium. You will remember that the equation for this is.
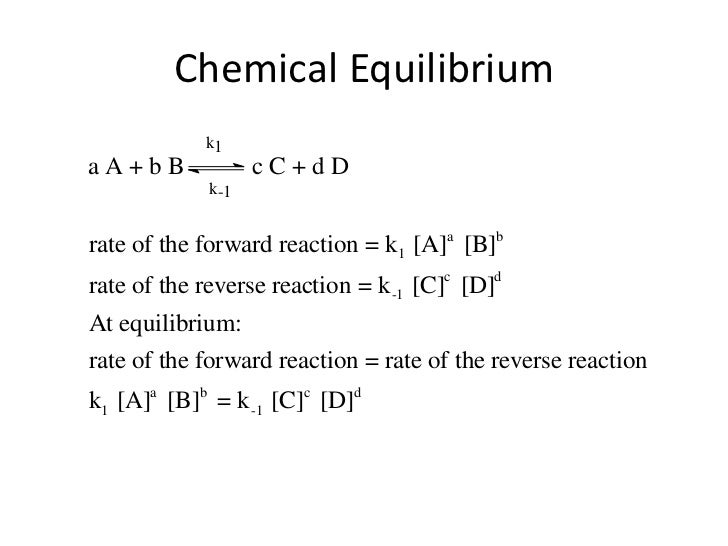
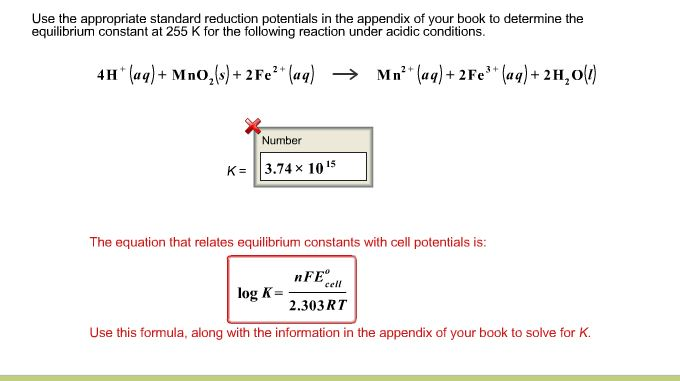
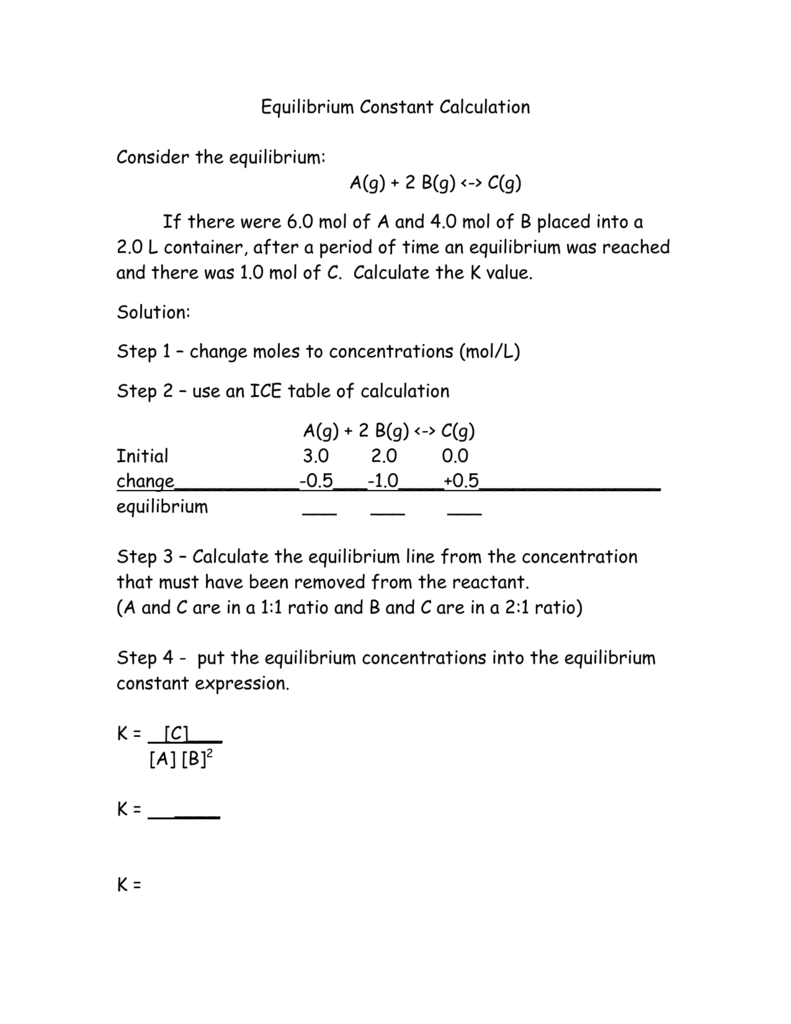
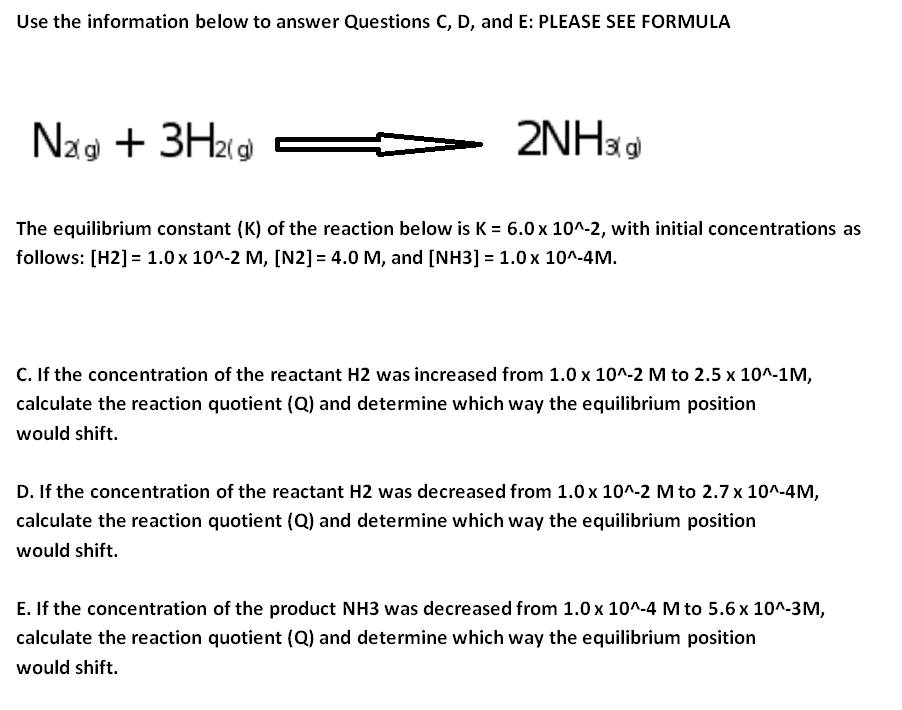
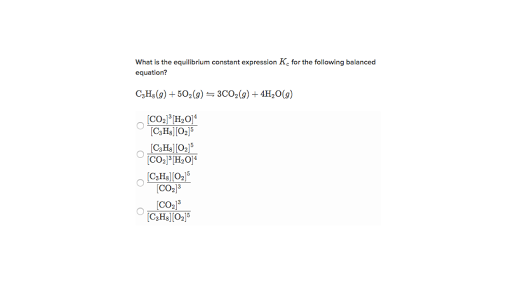
The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency towards further changefor a given set of reaction conditions the equilibrium constant is independent of the initial analytical concentrations of the. The equilibrium constant k. To calculate an equilibrium concentration from an equilibrium constant an understanding of the concept of equilibrium and how to write an equilibrium constant is required.
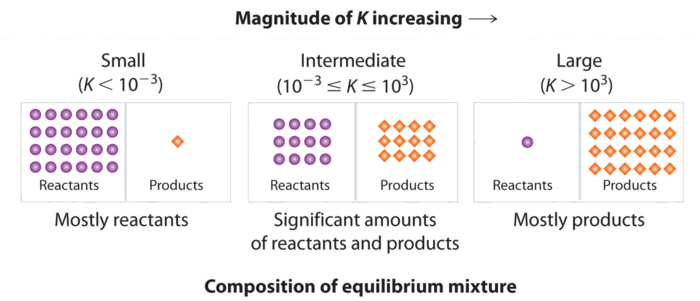
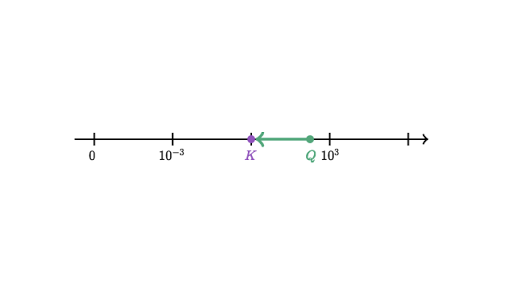
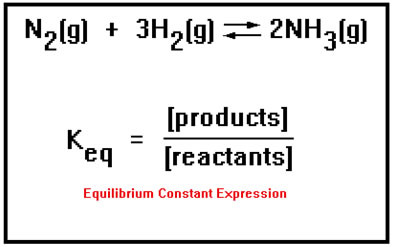
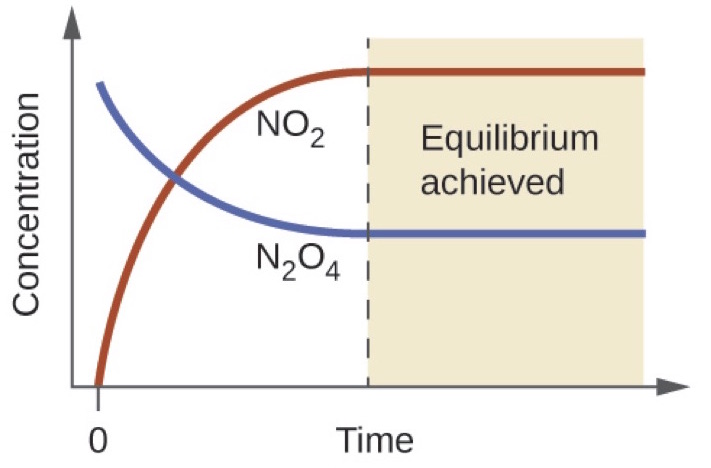
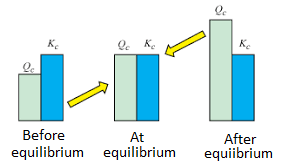
Chemical equilibrium is the state in which the reactants and products have no net change over time. How to calculate k and how to use k to determine if a reaction strongly favors products or reactants at equilibrium. This is the currently selected item.
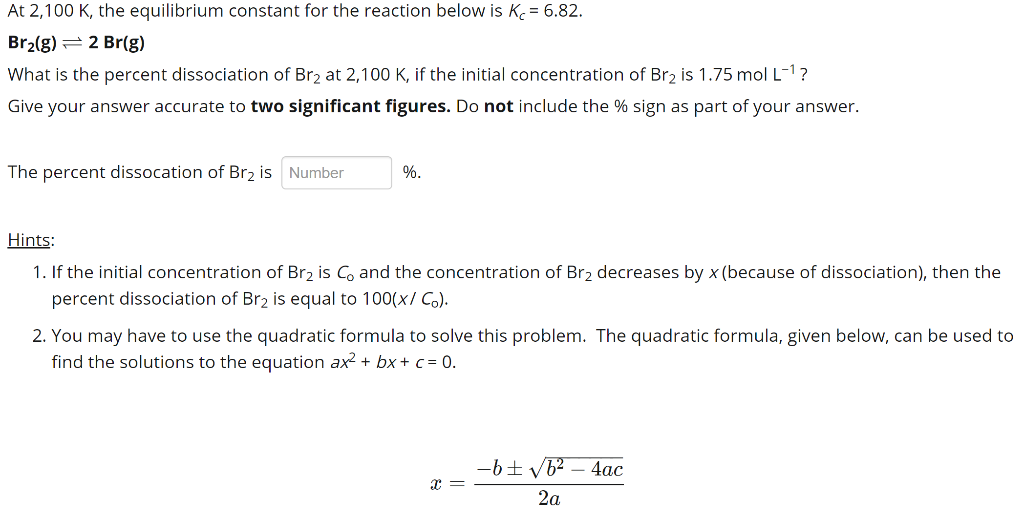
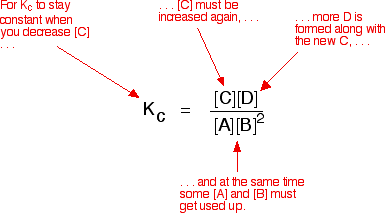
Small x approximation for large kc. You will find a link to. This expression k b is based on the general form for k c.
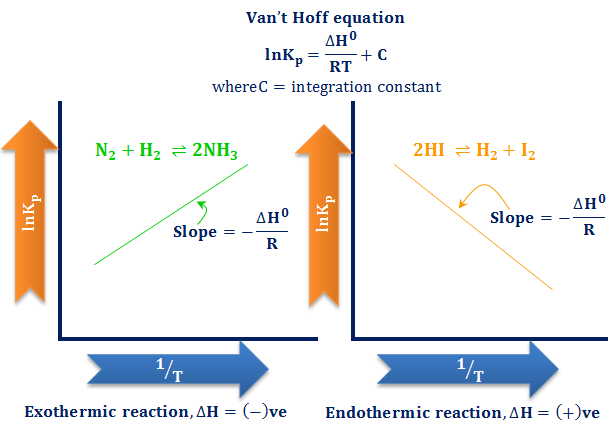
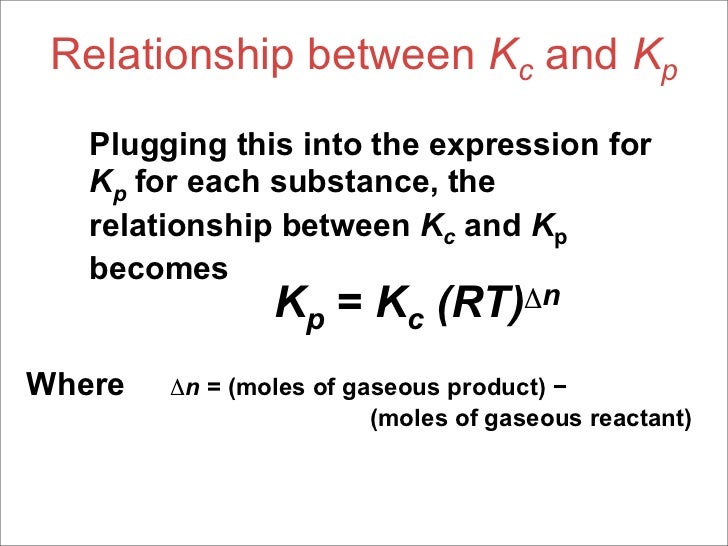
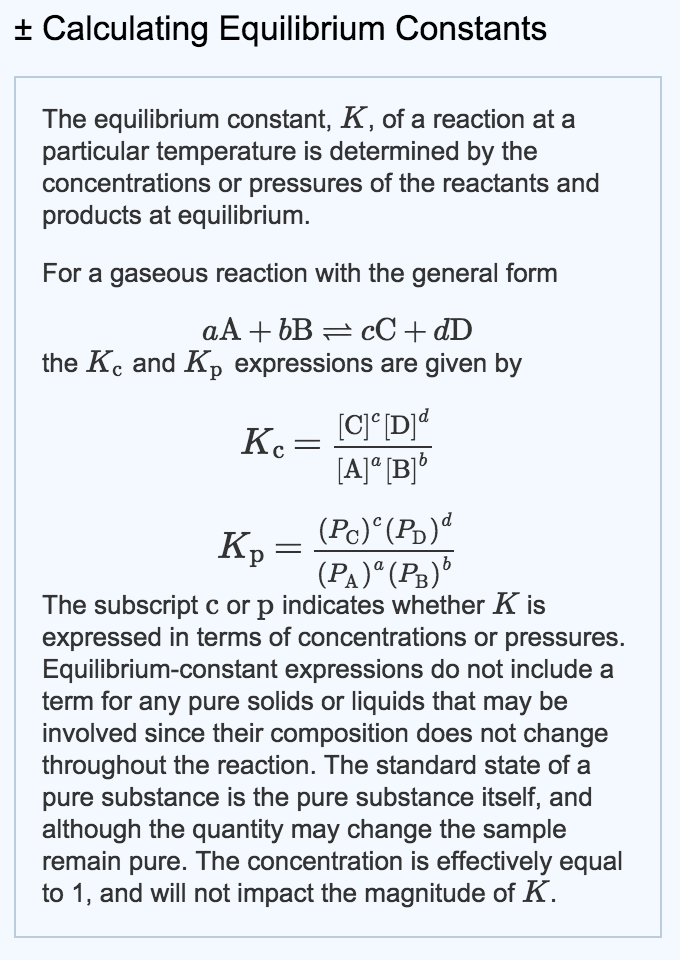
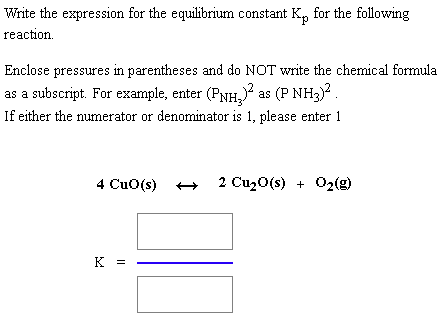
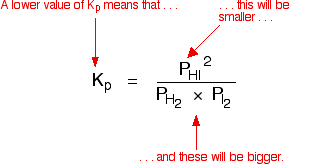
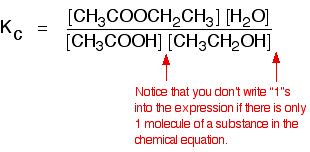
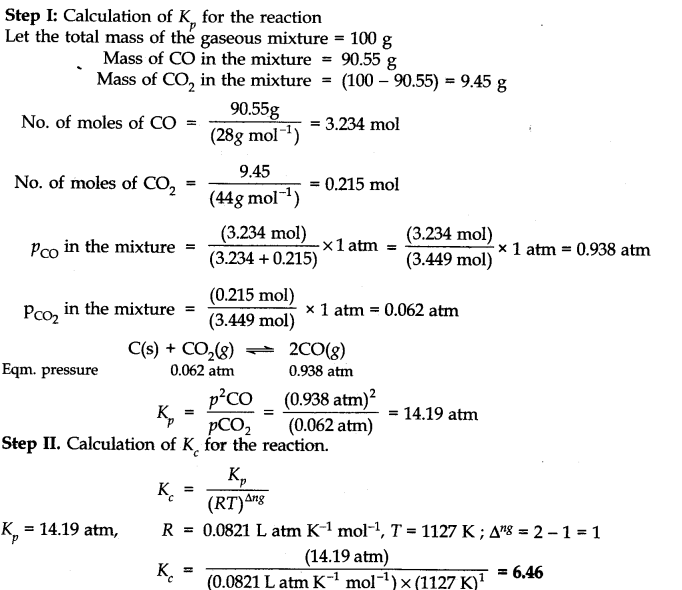
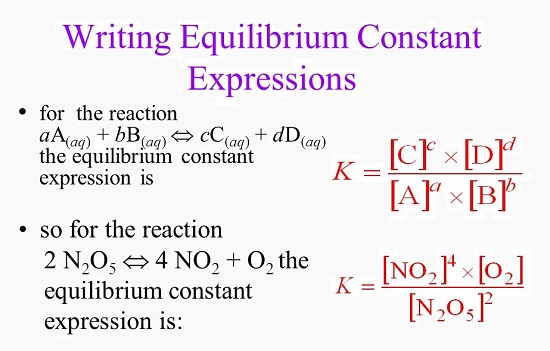
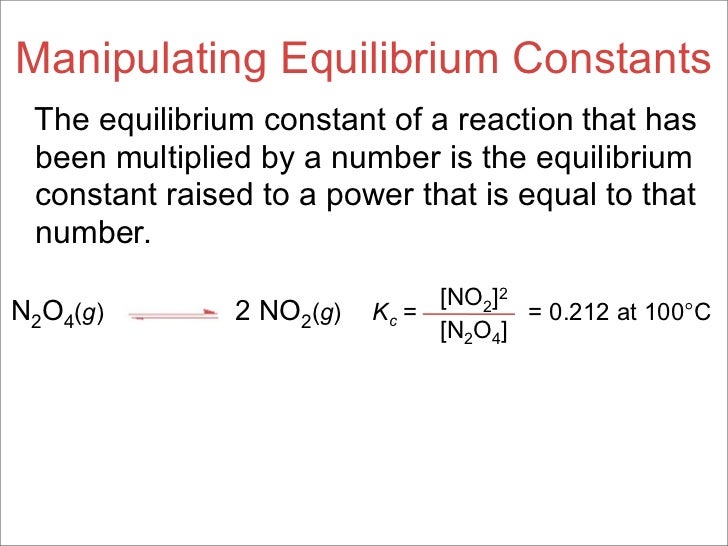
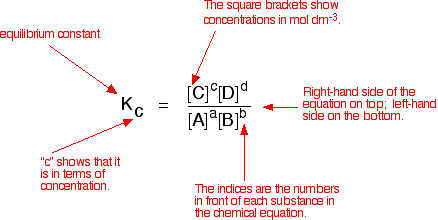
This time the k c expression will include some visible powers. There is another equilibrium constant called k p which is more frequently used for gases. Although everything is present as a gas you still measure concentrations in mol dm 3.
This is when the forward and reverse reactions occur at equal rates. Calculating equilibrium constant kp using partial pressures. Equilibrium constant definition.
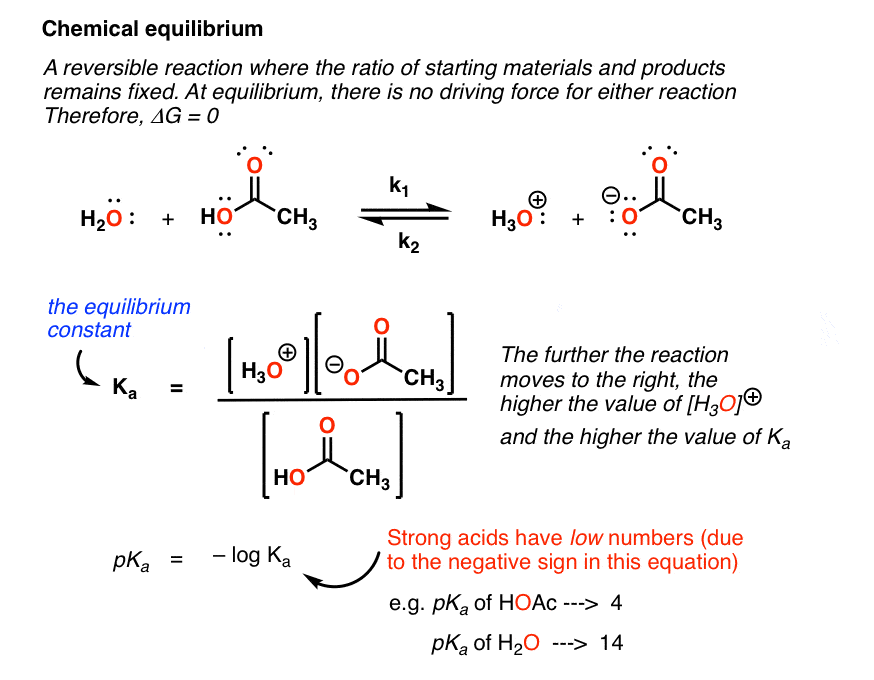
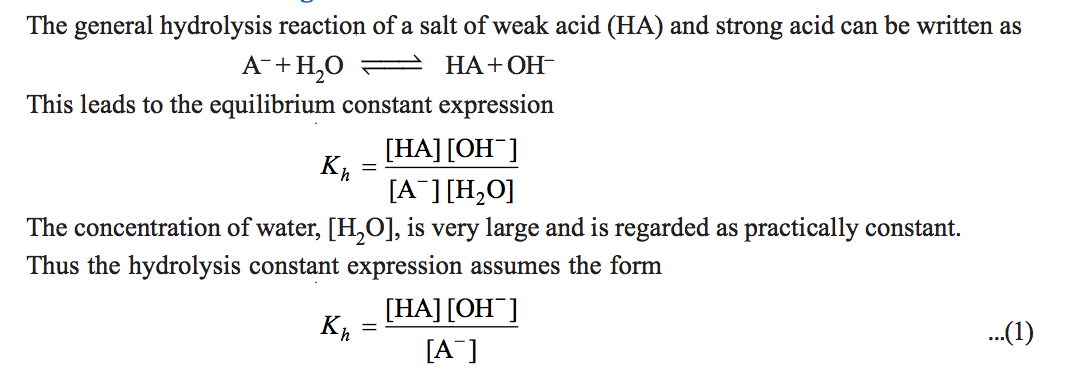
The designation k b is used to indicate that it is the equilibrium constant for the reaction of a base with water. Equilibrium is a state of dynamic balance where the ratio of the product and reactant concentrations is constant. The equation representing the ionization of any weak acid b and the equilibrium expression k b are shown below.
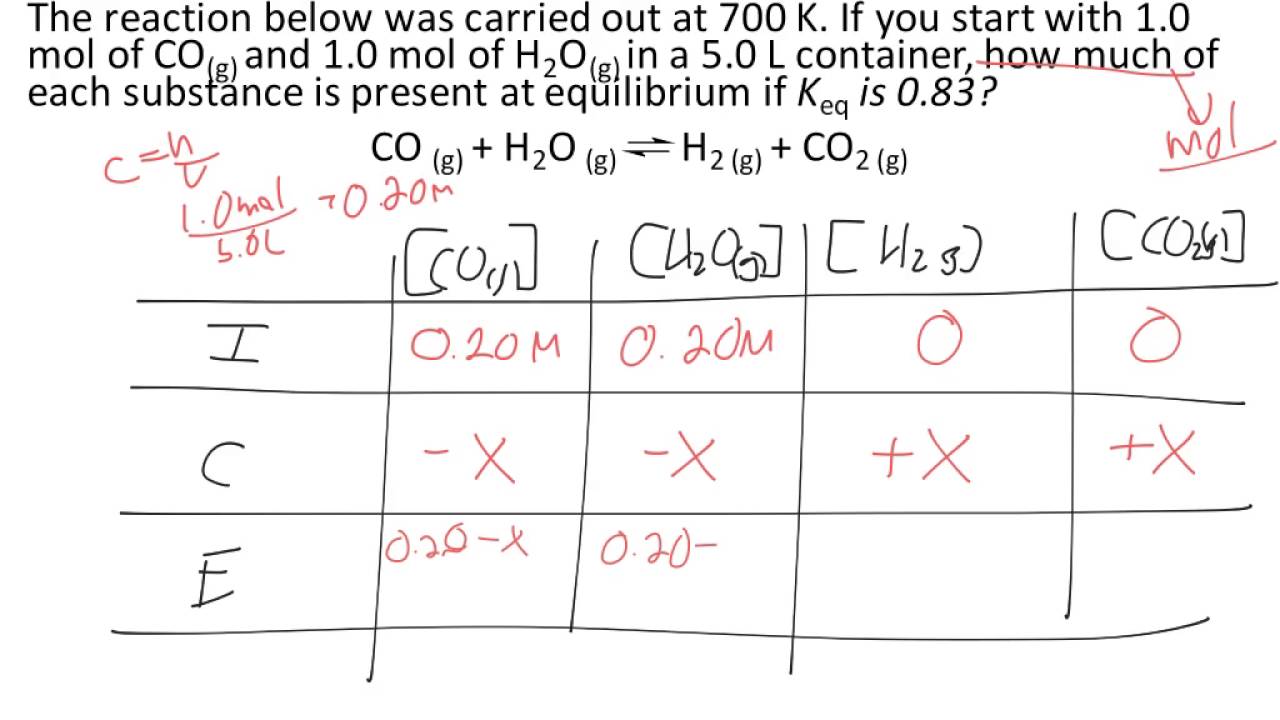
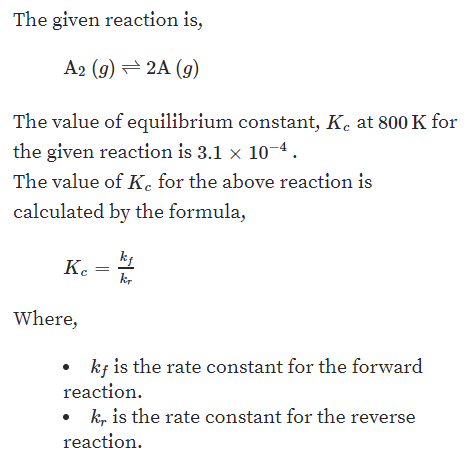
Reversible reactions equilibrium and the equilibrium constant k. This state of equilibrium can be described by the equilibrium constant k. The page assumes that you are already familiar with the concept of an equilibrium constant and that you know about k c an equilibrium.
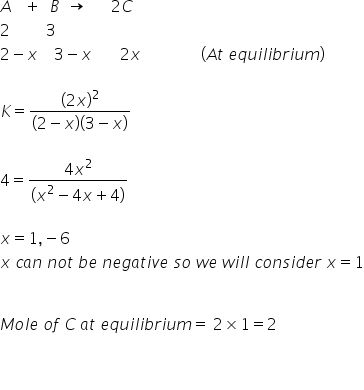
For A Reaction A B 2c 2 Moles Of A And 3 Moles Of B Are Allowed To React If Equilibrium Constant Is 4 At 400 C Then The Mole Of C
www.topperlearning.com