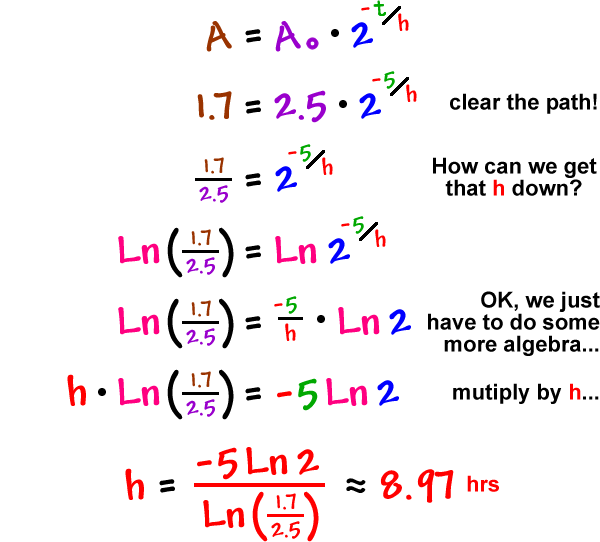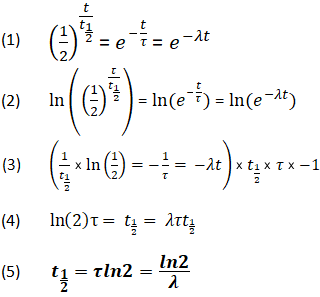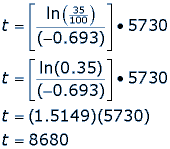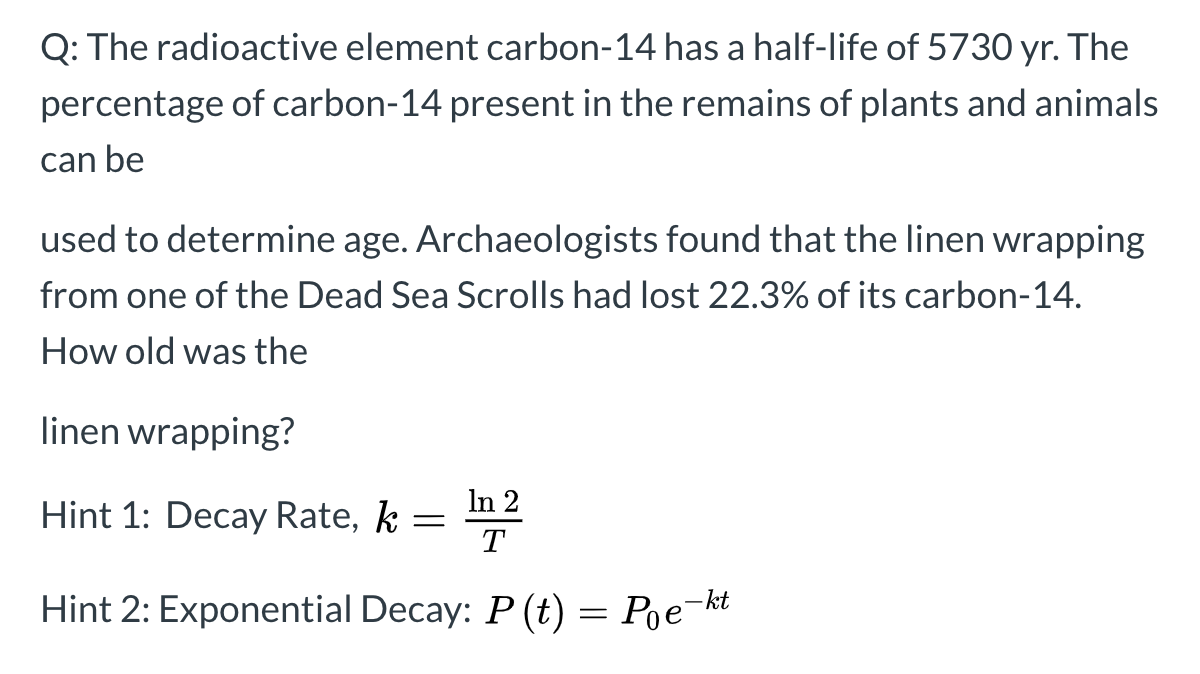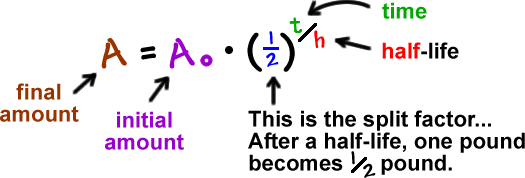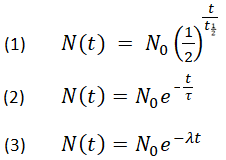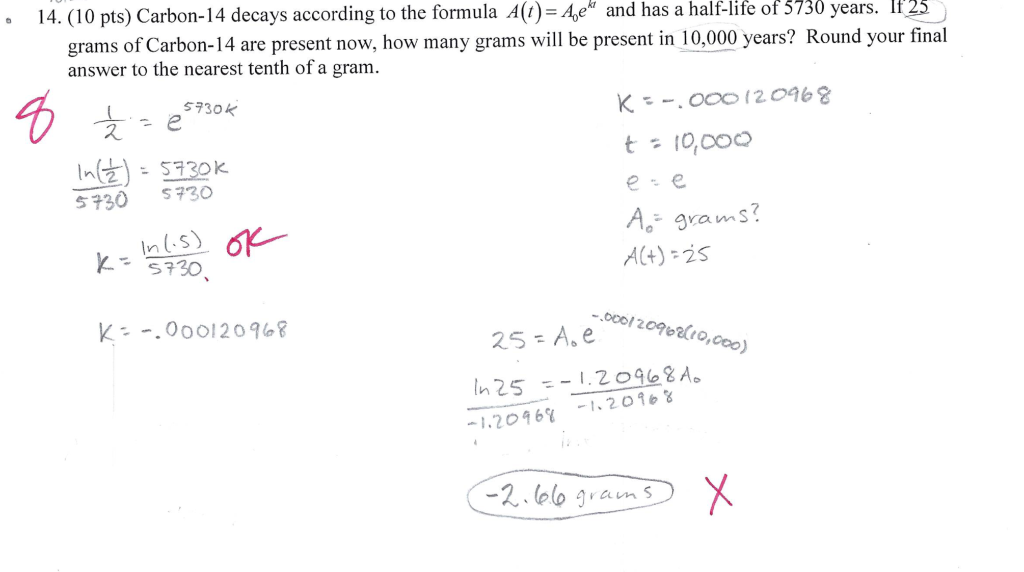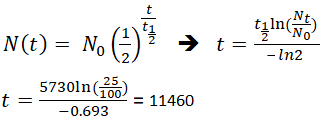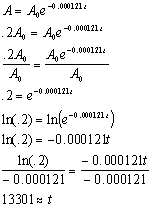Half Life Formula Carbon 14
Radioactive decay and detection.
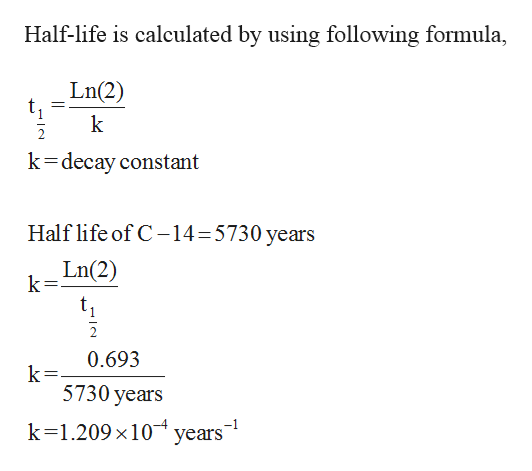
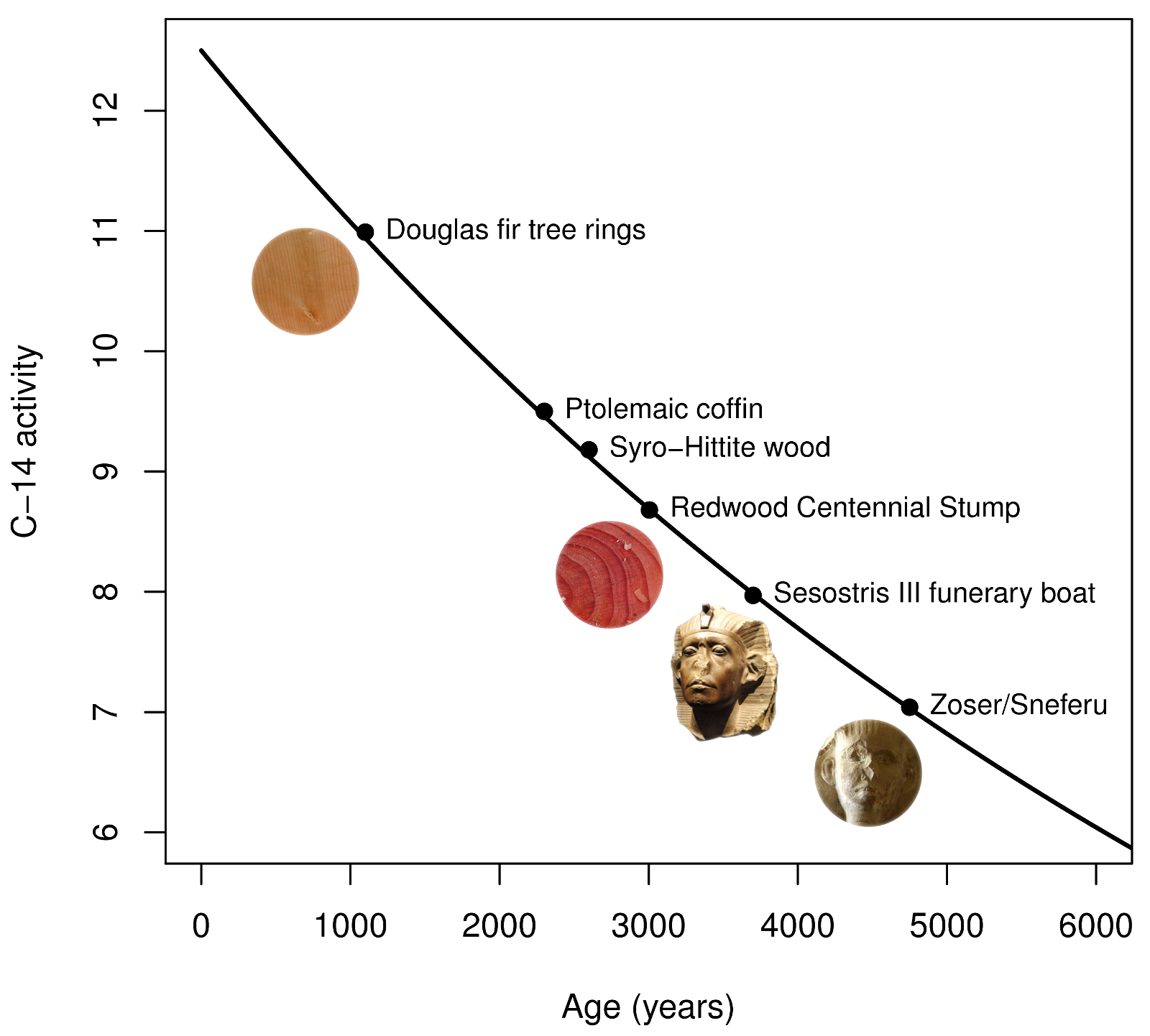
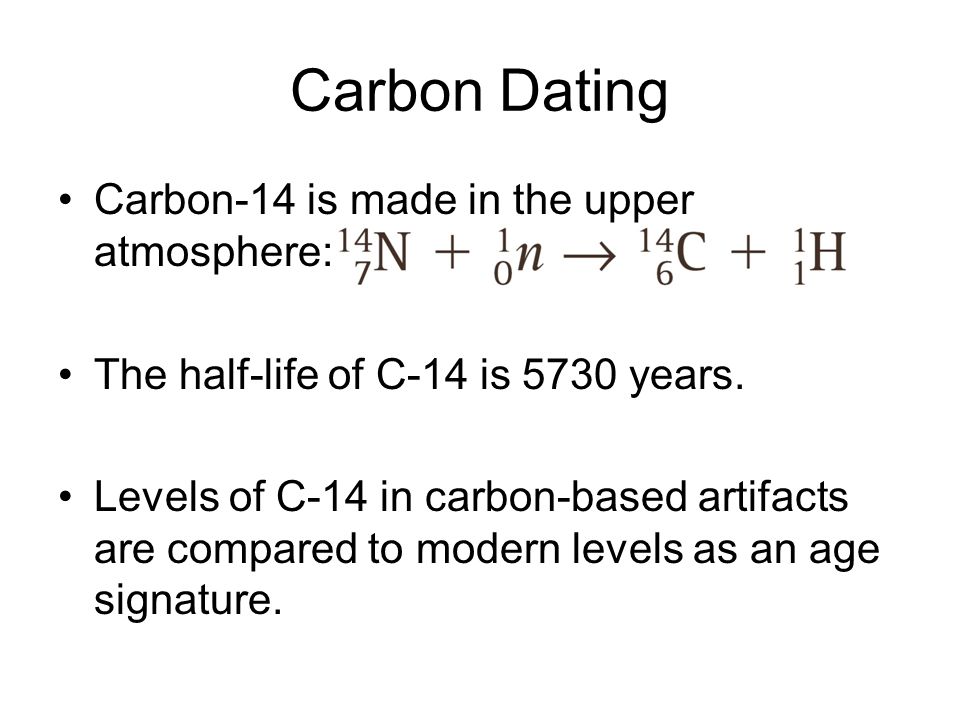
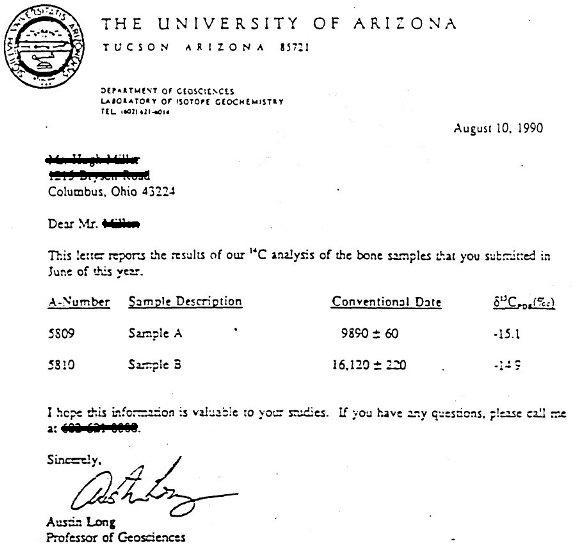
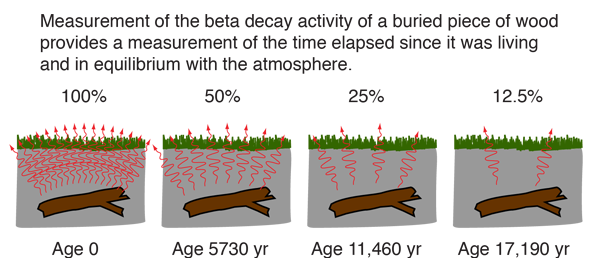
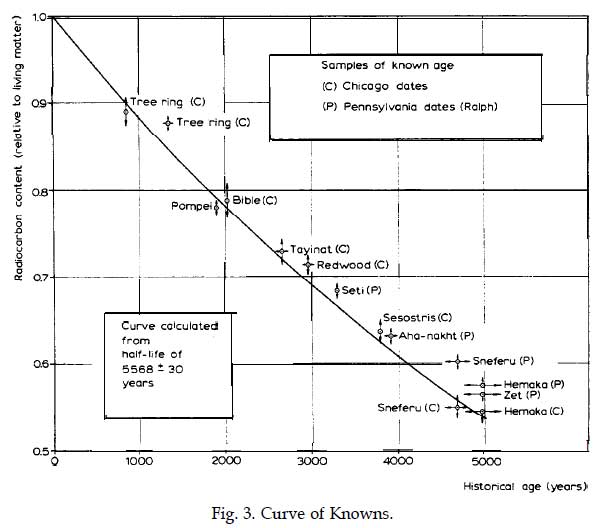
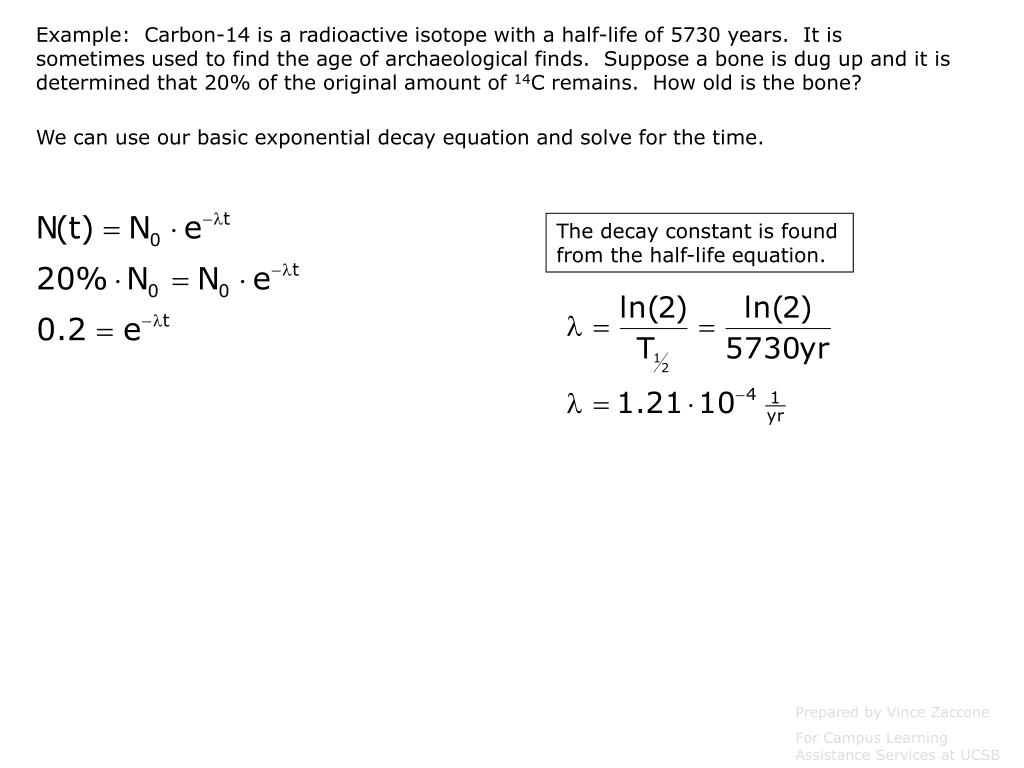
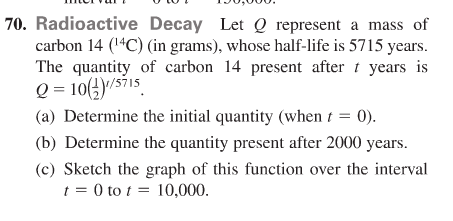
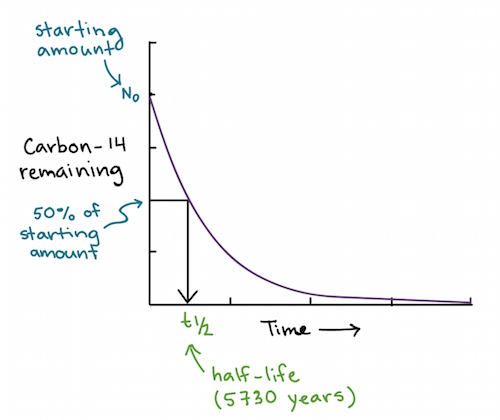
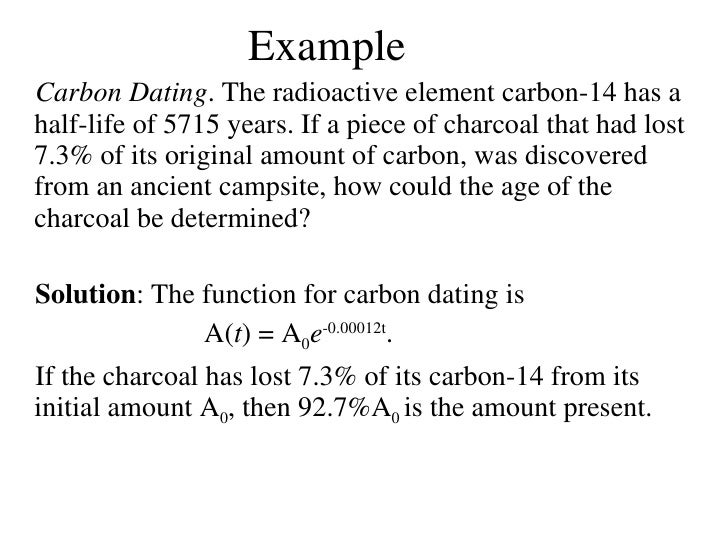
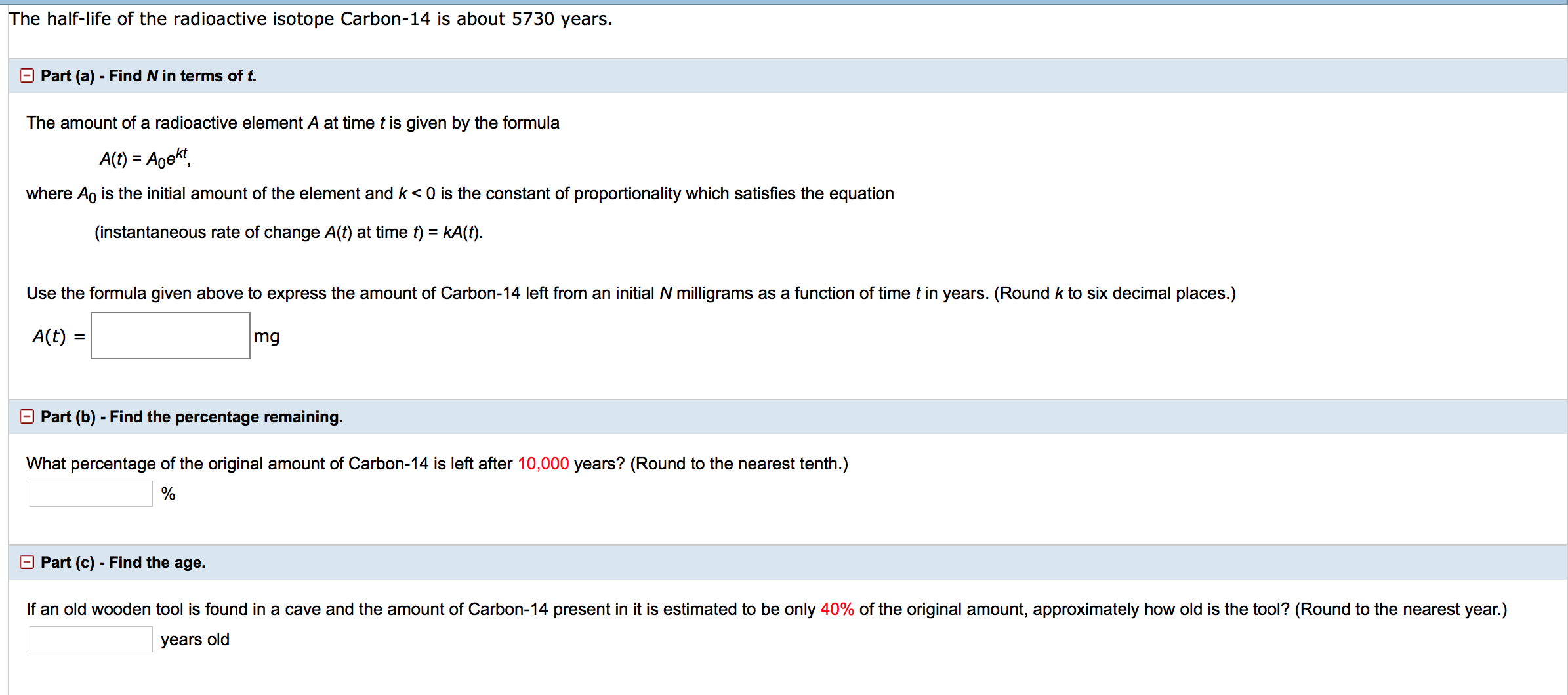
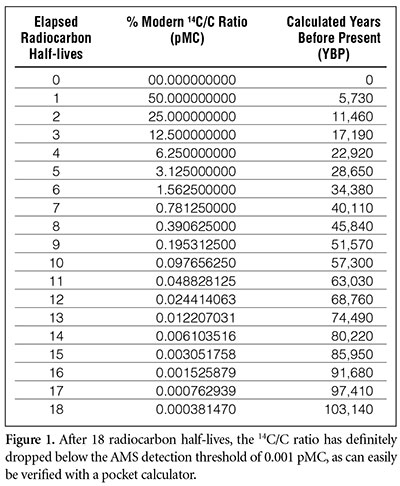
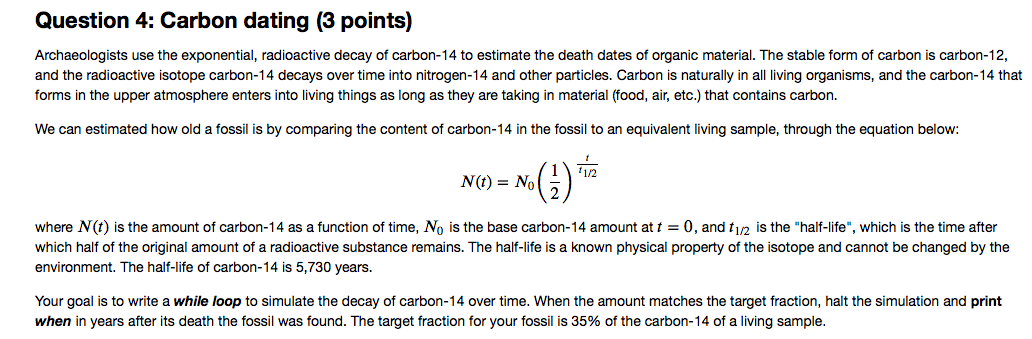
Half life formula carbon 14. The half life of a radioactive isotope describes the amount of time that it takes half of the isotope in a sample to decay. The process of carbon 14 dating was developed by william libby and is based on the fact that carbon 14 is constantly being made in the atmosphere. The halflife of carbon 14 is 5730 30 years and the method of dating lies in trying to determine how much carbon 14 the radioactive isotope of carbon is present in the artifact and comparing it to levels currently present.
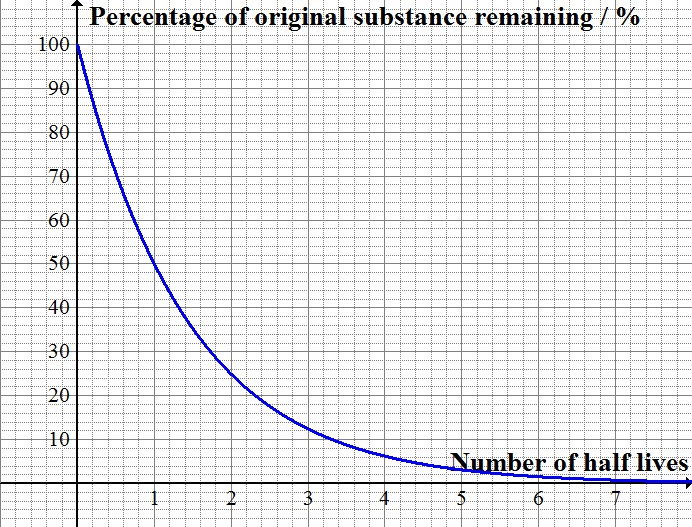
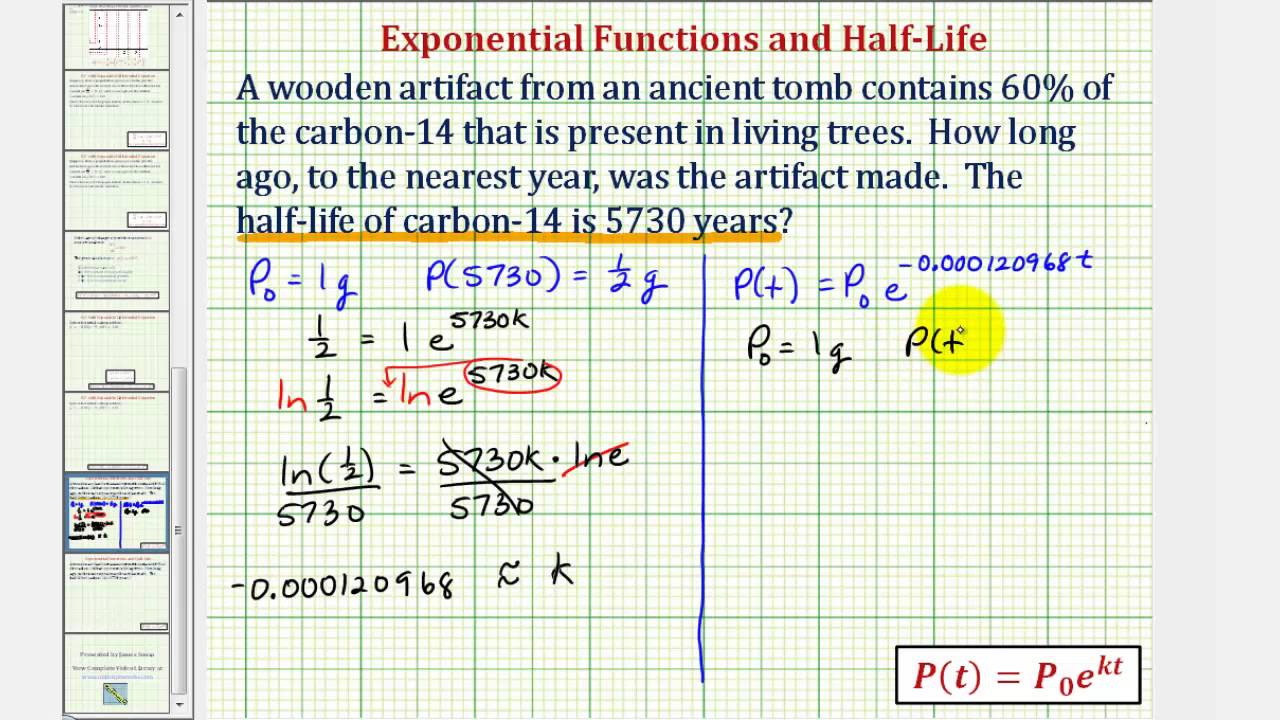
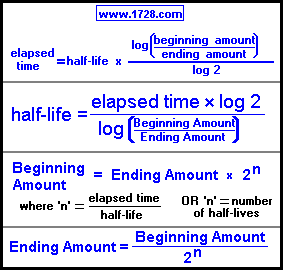
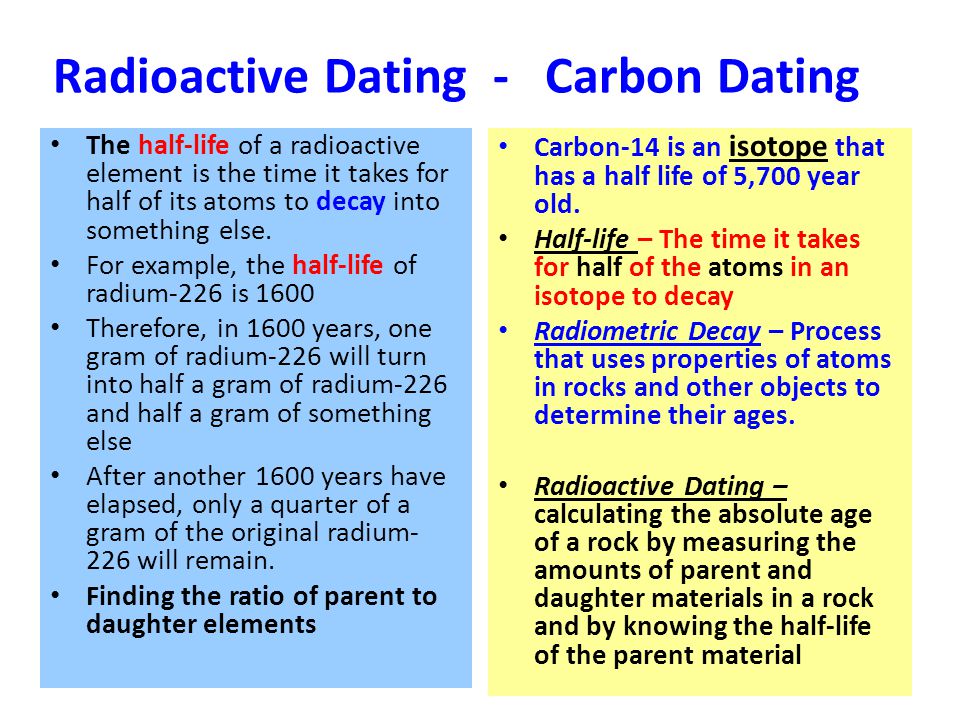
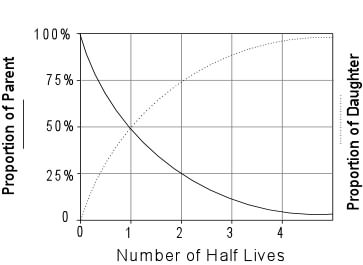
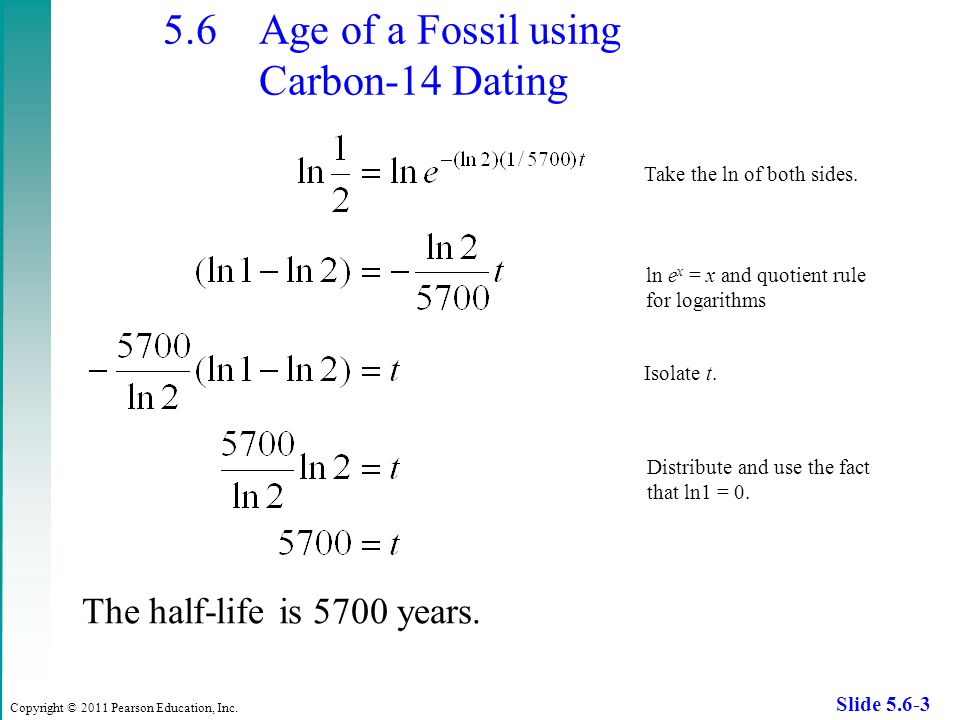
This half life is a relatively small number which means that carbon 14 dating is not particularly helpful for very recent deaths and deaths. Where ln nfno the natural logarithm of the percent carbon 14 in the sample compared to the percent carbon 14 in living tissue and t12 the half life of carbon 14 5700 years. For example 20 units of a substance with a half life of 5 years will have 10 units remaining after 5 years.
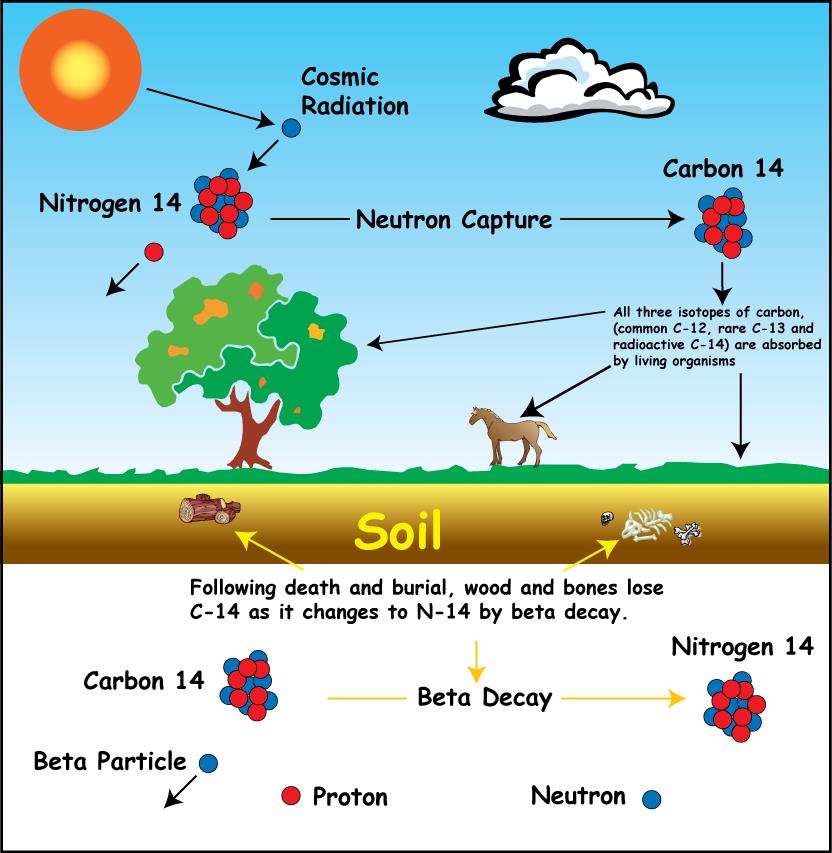
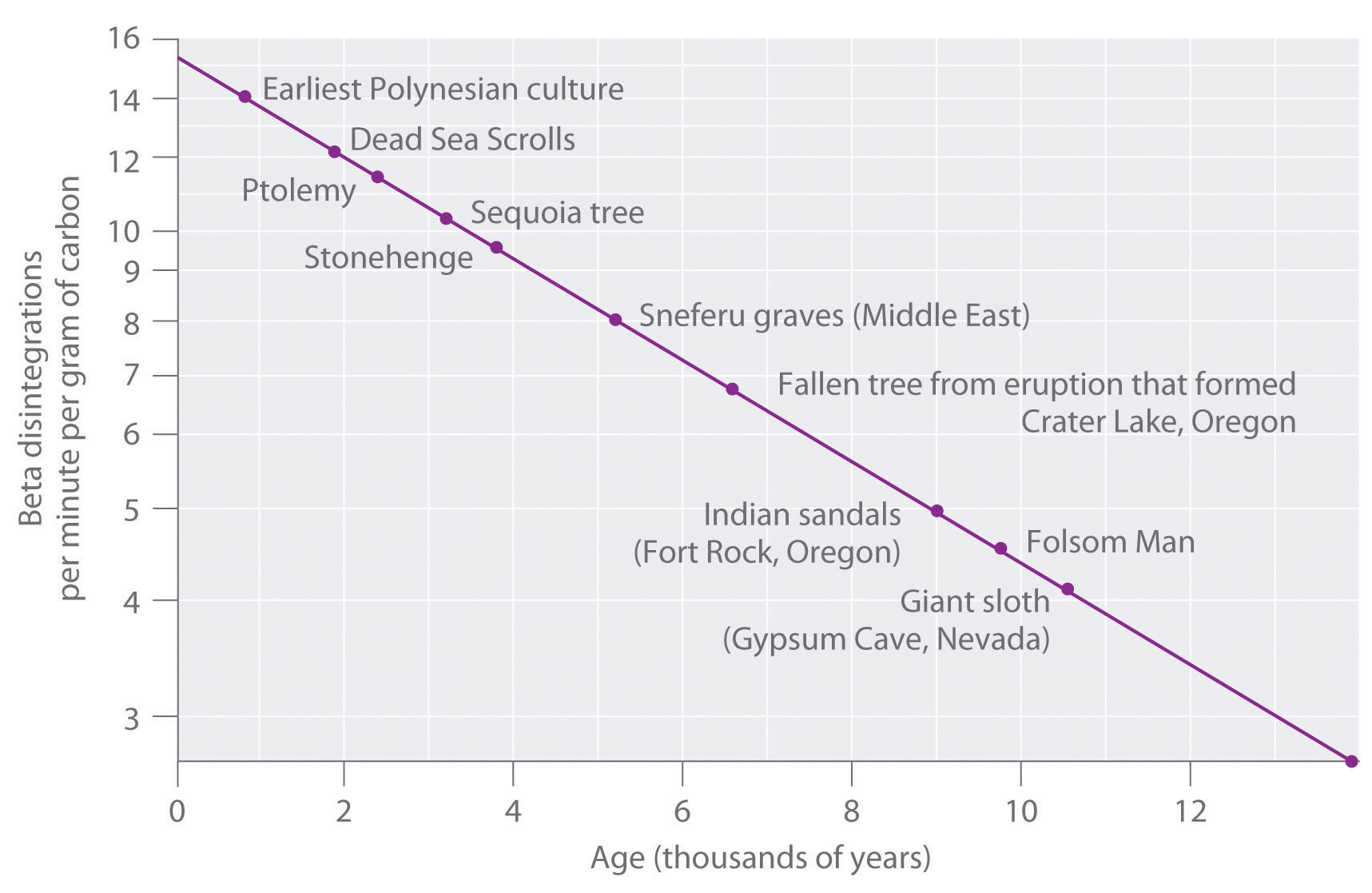
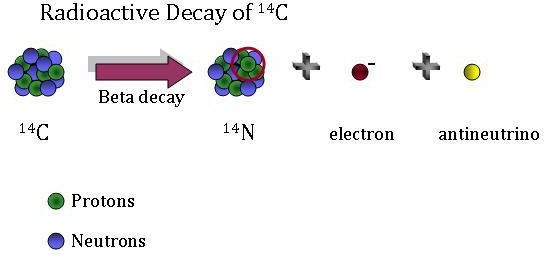
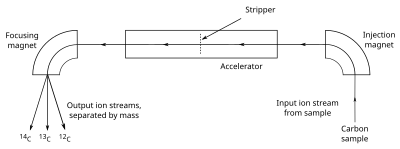
Carbon dating has given archeologists a more accurate method by which they can determine the age of ancient artifacts. 14 6 c 14 7 n e n e. The longer it decays the less carbon is present which can be used to date the organism based on carbons half life.
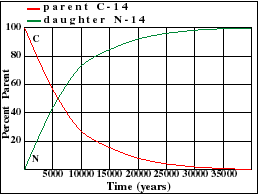
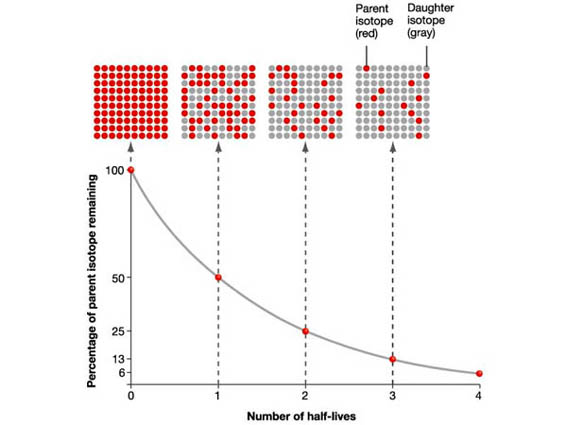
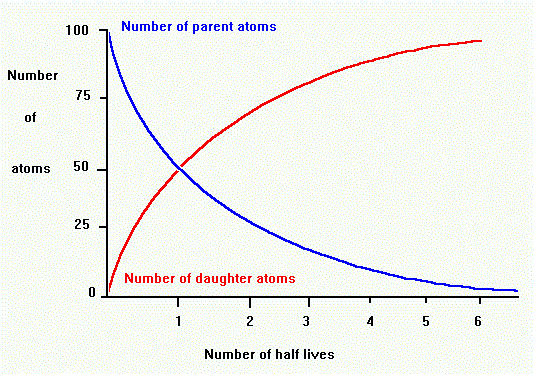
The carbon 14 decays with its half life of 5700 years while the amount of carbon 12 remains constant in the sample. Carbon 14 which decays and carbon 12 which stays constant. The emitted beta particles have a maximum energy of 156.
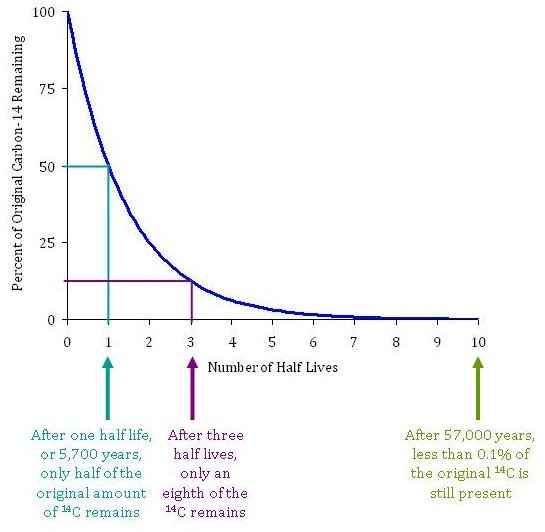
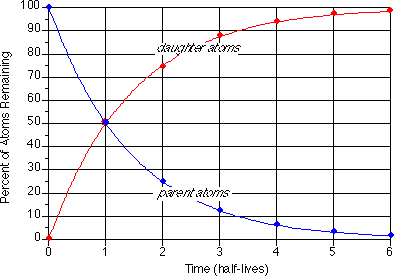
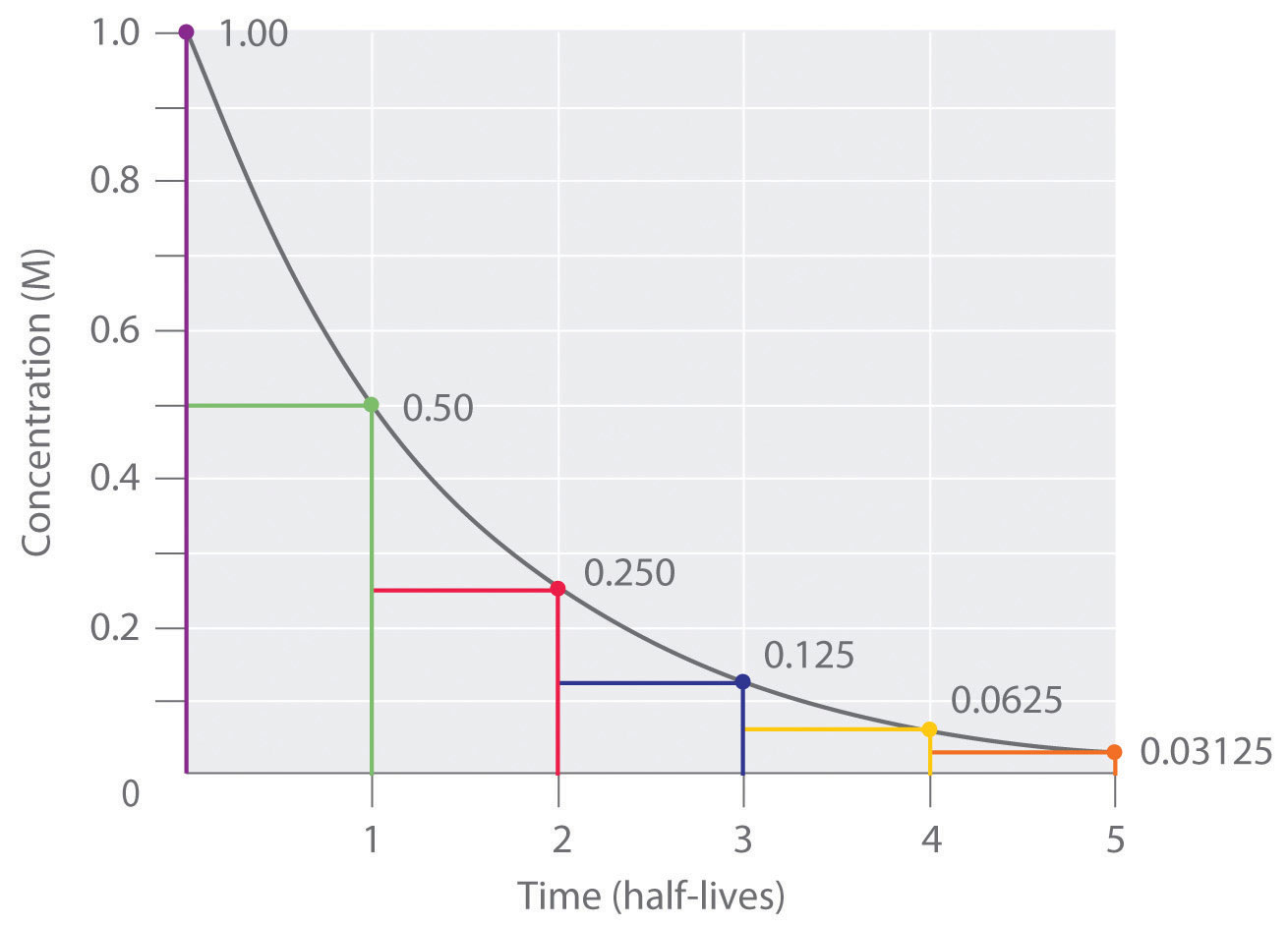
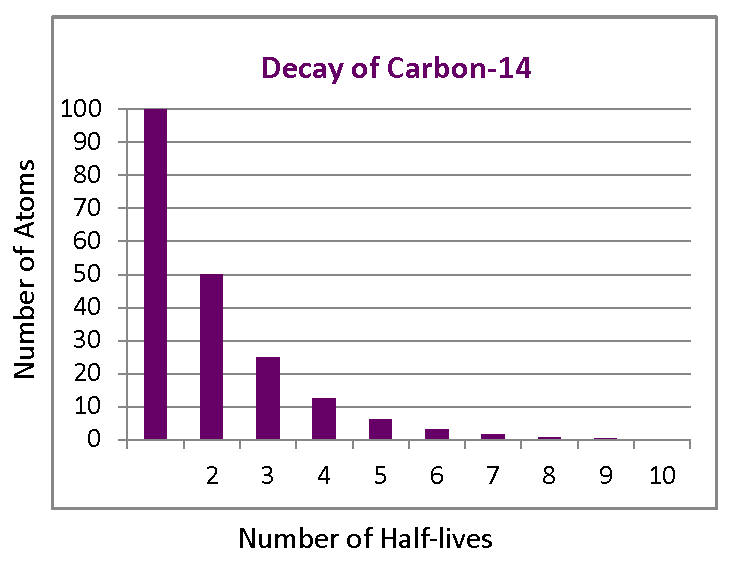
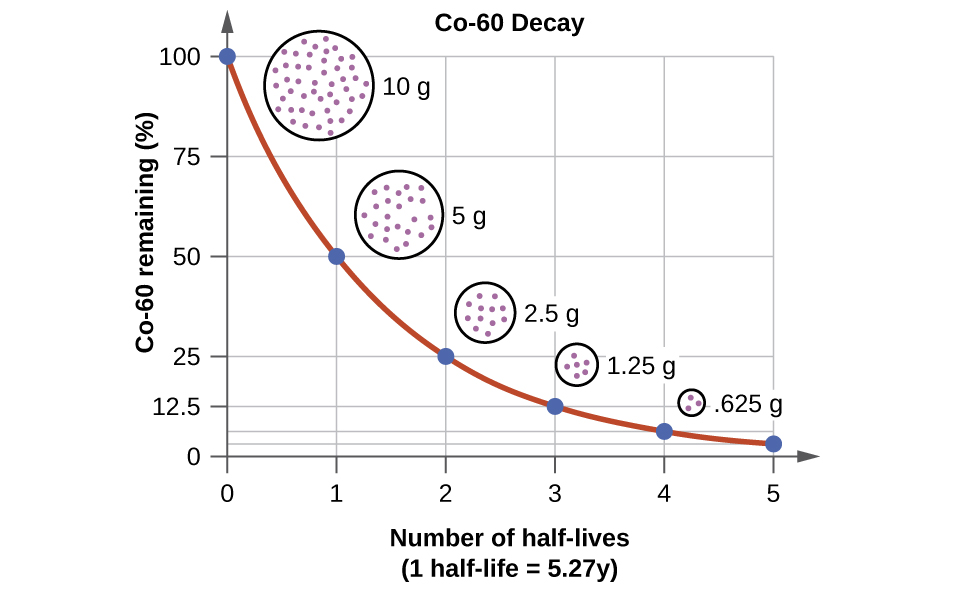
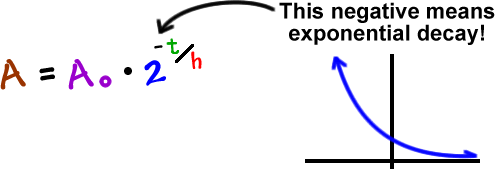
Carbon 14 goes through radioactive beta decay. The half life is the time it takes for 12 of a radioactive substance to decay. In this example carbon 14 has undergone 3 half lives.
If you wanted to date a fossil first you would determine the percent carbon 14 it contained compared to a living sample. By looking at the ratio of carbon 12 to carbon 14 in the sample and comparing it to the ratio in a living organism it is possible to determine the age of a formerly living thing fairly precisely. In the case of radiocarbon dating the half life of carbon 14 is 5730 years.
X research source technically there are 2 types of carbon.
Exponentials Logarithms Cool Math Algebra Help Lessons Radioactive Decay And Decibel Levels
www.coolmath.com