What Would Be The Mass Of 976 X 1022 Formula Units Of Srcl2

International Critical Tables Of Numerical Data Physics Chemistry And Technology 1st Electronic Edition Cement Glasses
www.scribd.com
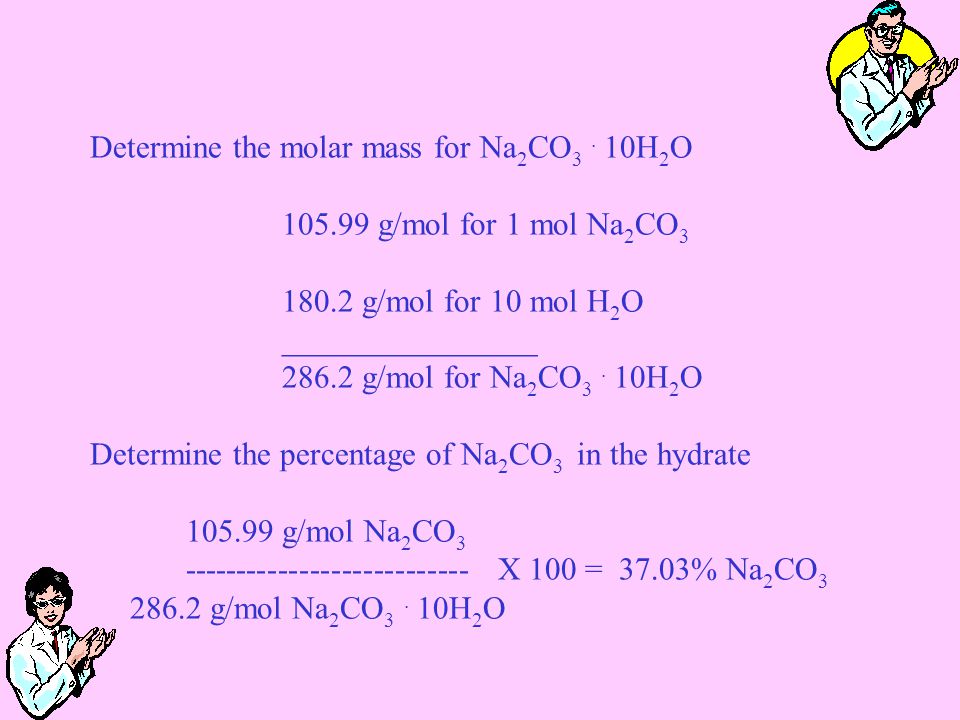
The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.

What would be the mass of 976 x 1022 formula units of srcl2. For sulphuric acid the molar mass of hydrogen is multiplied by 2 sulphur is multiplied by 1 and oxygen is multiplied by 4. Finding molar mass starts with units of grams per mole gmol. Once you know how many moles you have you can use calcium chlorides molar mass to convert those to grams.
One mole of carbon is 6022 x 10 23 atoms of carbon avogadros numberthis relation is then used to convert a carbon atom to grams by the ratio. Mass in kilograms of 46 x 1021 molecules of no2 b. Mass in milligrams of 288 x 1022 formula units of bino33 5h2o this means that its a superscript.
When the moles have been converted into grams it is easier to carry out calculations using the metric system. Thus the molar mass of sulphuric acid is equal to 21008 3206 416 9808 grams. Number of h ions in 582 g of srh2 d.
Finally multiply by 1000 to get mg. Then multiply by 258 x 1022. Moles of ci atoms in 00615 g of c2h4cl2 c.
1000 x 10 6 g. What must be the mass of this sample. The mass of an atom is so small it is rounded to 0 amu.
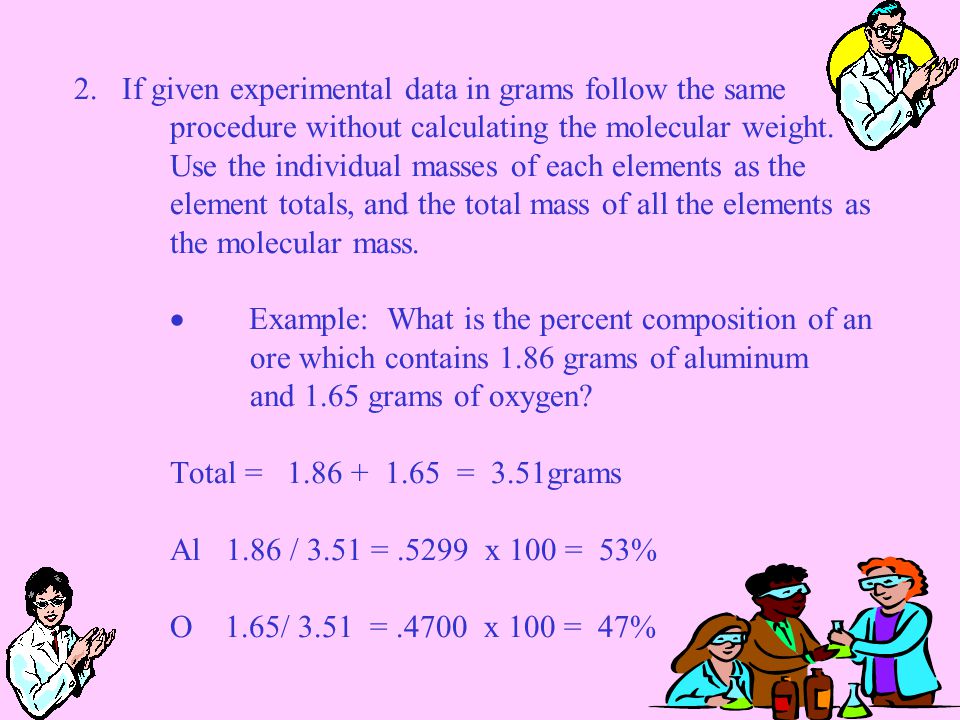
To calculate the mass of a single atom first look up the atomic mass of carbon from the periodic table. This number 1201 is the mass in grams of one mole of carbon. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance.
441 x 1025 formula units of strontium hydrogen carbonate. 555 g what you need to do here is use avogadros number to calculate how many moles of calcium chloride cacl2 are equivalent to that many formula units of this salt. 2197 1 calculate the molarity of bromide ions in 250.
Ml of a solution containing 259 g nabr and 0155 moles of hbr. Finding molar mass starts with units of grams per mole gmol. A sample of alcl 3 contains a total of 4515 x 10 27 atoms of chlorine.
Answer choices 1622 g. So avogadros number which essentially acts as the definition of a mole tells you that one mole of any ionic. Mass in kilograms of 358 mol of cucl2 2h2o f.
2500 x 10 5 g. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. Total number of ions in 381 g of srf2 e.
Take 485067g and divide by 602 x 1023 1 mole.

Diffusion Coefficients And Accessible Porosity For Hto And 36cl In Compacted Febex Bentonite Request Pdf
www.researchgate.net

Iit Jee Super Course In Chemistry Vol 1 Physical Chemistry By Trishna Knowledge Systems Z Lib Org Pdf Concept Test Assessment
www.scribd.com