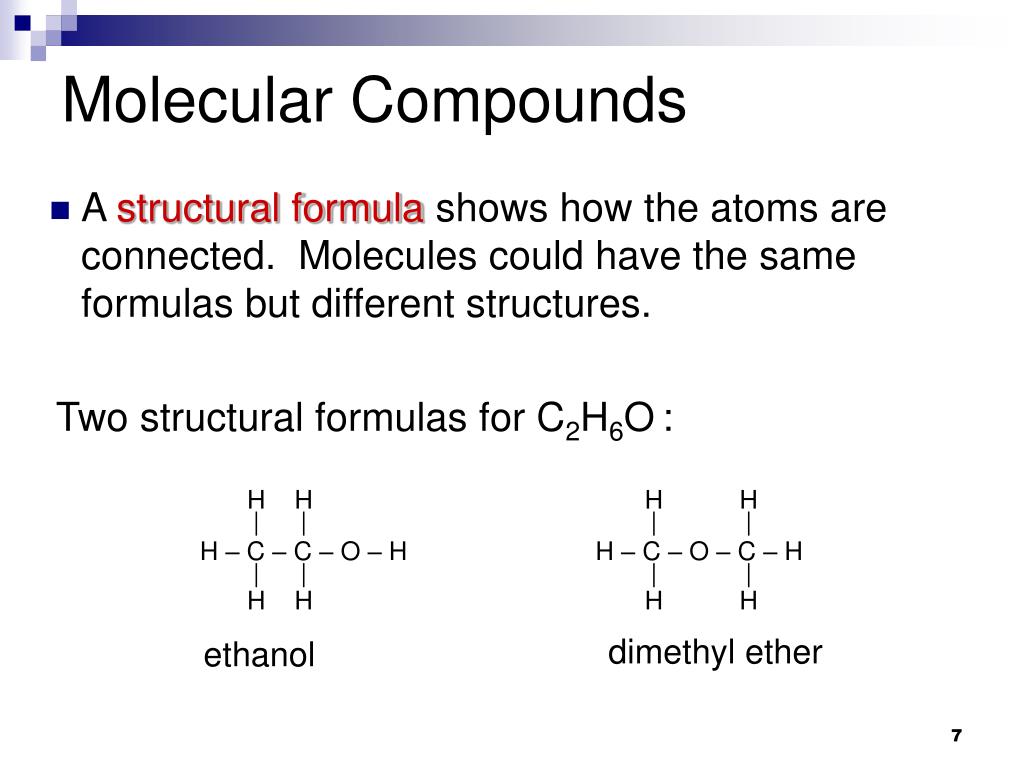
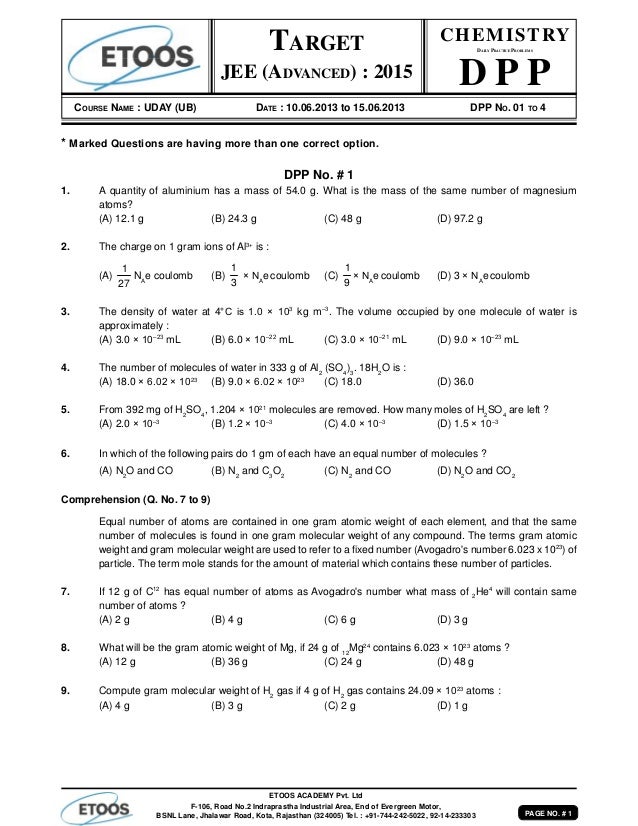
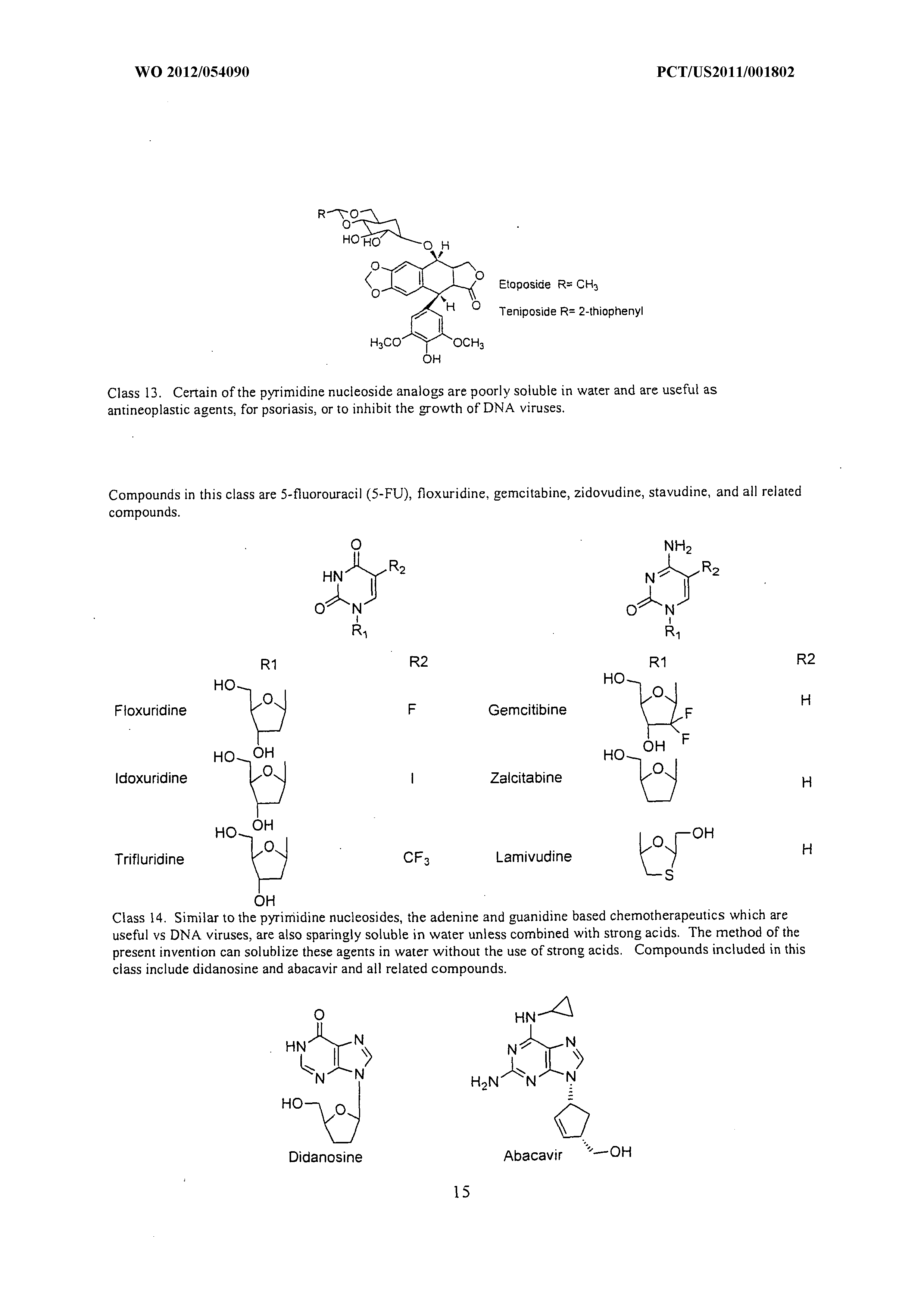
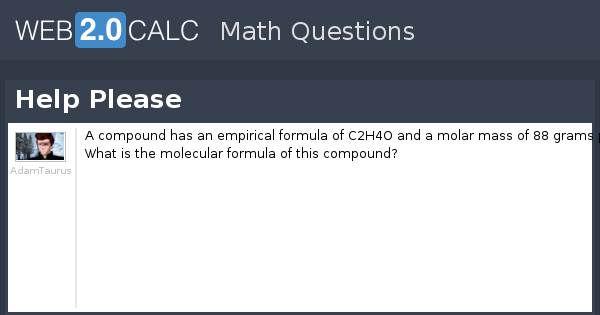
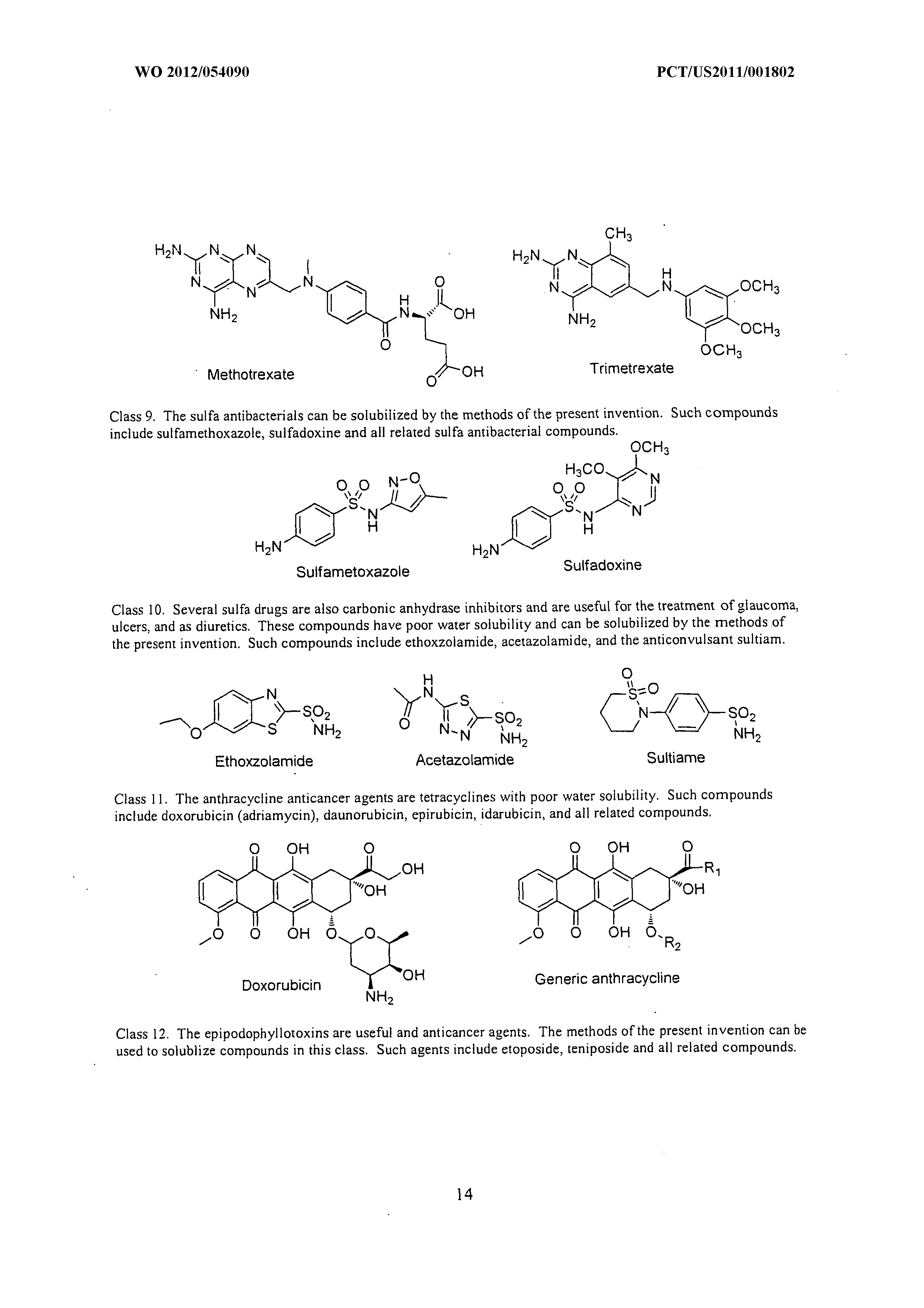
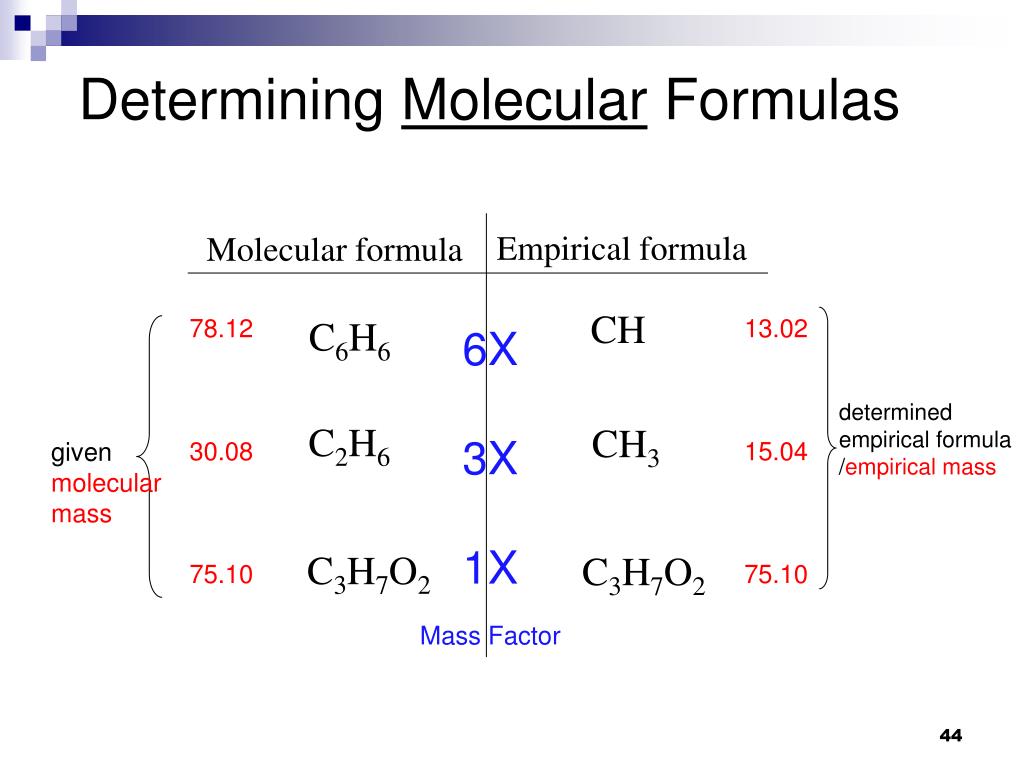
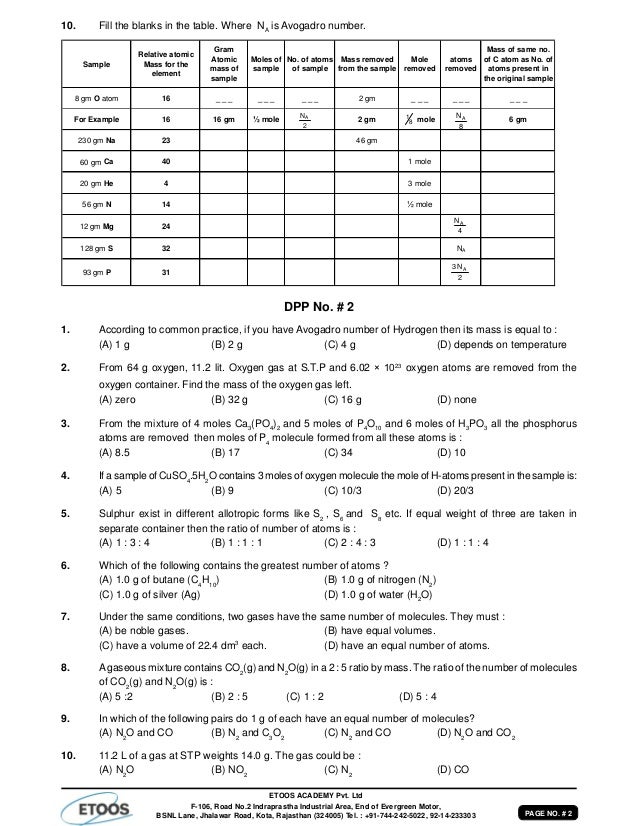
What Is The Mass In Grams Of 1 Mole Of A Compound Whose Formula Is C3h7o2

Analytical Profiles Of Drug Substances Excipients And Related Methodology Volume 31 Mass Spectrometry X Ray Crystallography
www.scribd.com
If the formula used in calculating molar mass is the molecular.
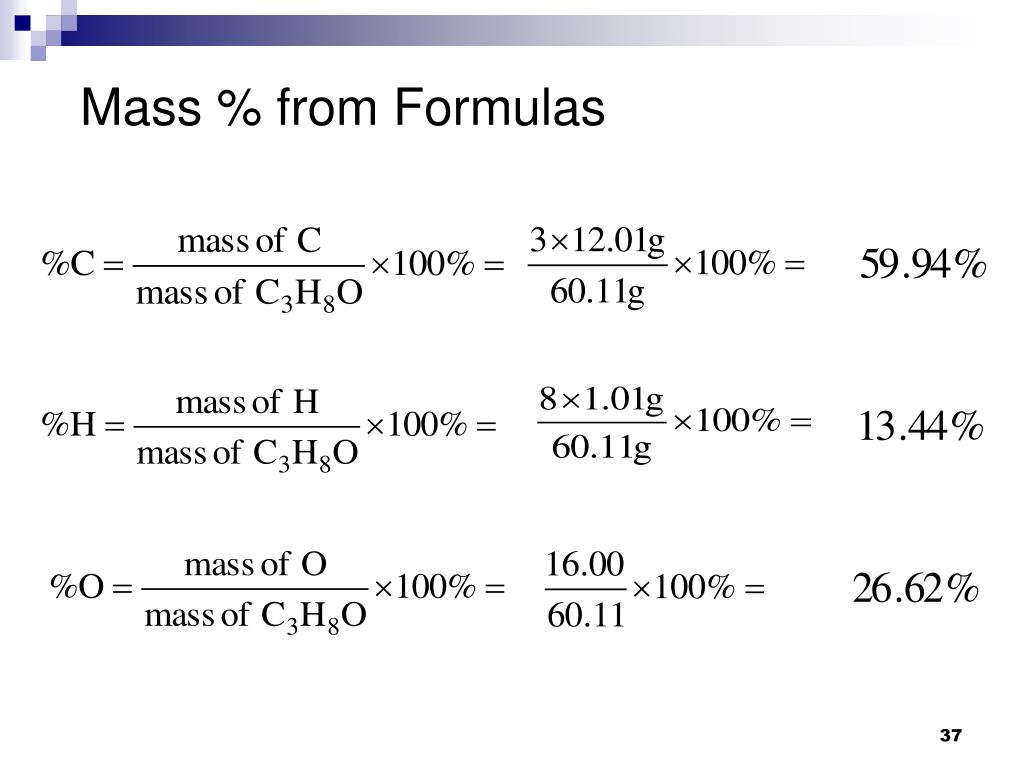
What is the mass in grams of 1 mole of a compound whose formula is c3h7o2. 75 g the net ionic equation for the reaction agno3 nacl agcl nano3 is. C one mole of a monatomic element has a mass equal to its atomic mass expressed in grams. That is the molar mass of a substance is the mass in grams per mole of 6022 10 23 atoms molecules or.
The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. The masses of 1 mole of different elements however are different since the masses of the individual atoms are drastically different. Which of the following statements about the mole is false.
B a mole of a monatomic element corresponds to one avogadros number of atoms. What is the molecular formula of a compound that weighs 46 gmol and analyzes to 522 c 348 o and 13 ha c2h5o2 b ch3o c c3h2o2 d c2h6o. What is the mass in grams of 1 mole of a compound whose formula is c3h7o2.
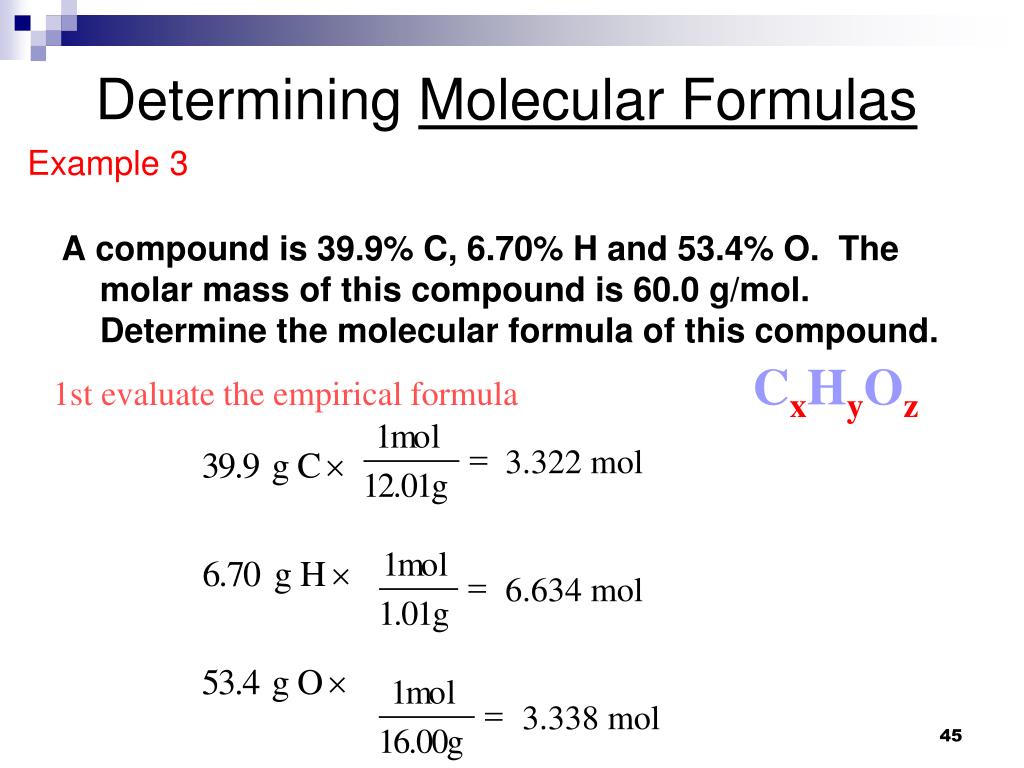
A the size of a mole of atoms has a reasonable mass. From this data determine the molecular formula. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance.
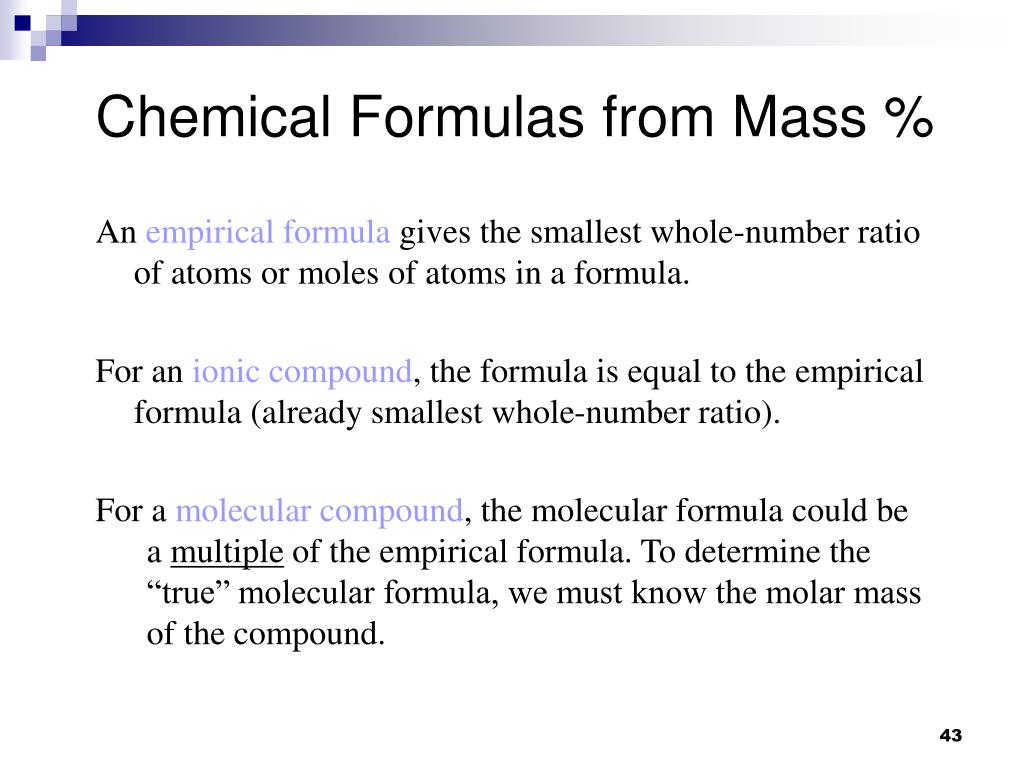
For a covalent molecular compound it is the mass of 1 mol of molecules of that compound. The molar mass of an element or compound is the mass in grams of 1 mole of that substance a property expressed in units of grams per mole gmol see figure 4. Use backchain dimensional analysis and show your work.
If a compounds density is 412 grams per liter then in 156 kilograms there are 3786 liters of the compound. For an ionic compound it is the mass of 1 mol of formula units. For a covalent molecular compound it is the mass of 1 mol of molecules of that compound.
D one mole of water contains 12 mole of oxygen atoms. For an element the molar mass is the mass of 1 mol of atoms of that element. For an ionic compound it is the mass of 1 mol of formula units.
Aspirin is commonly used as an analgesic antipyretic and an anti inflammatory medication. 1 liter is equal to 001 cubic meters. For an element the molar mass is the mass of 1 mol of atoms of that element.
What is the molecular formula for a compound whose empirical formula is ch and molar mass is 7911 gmol.
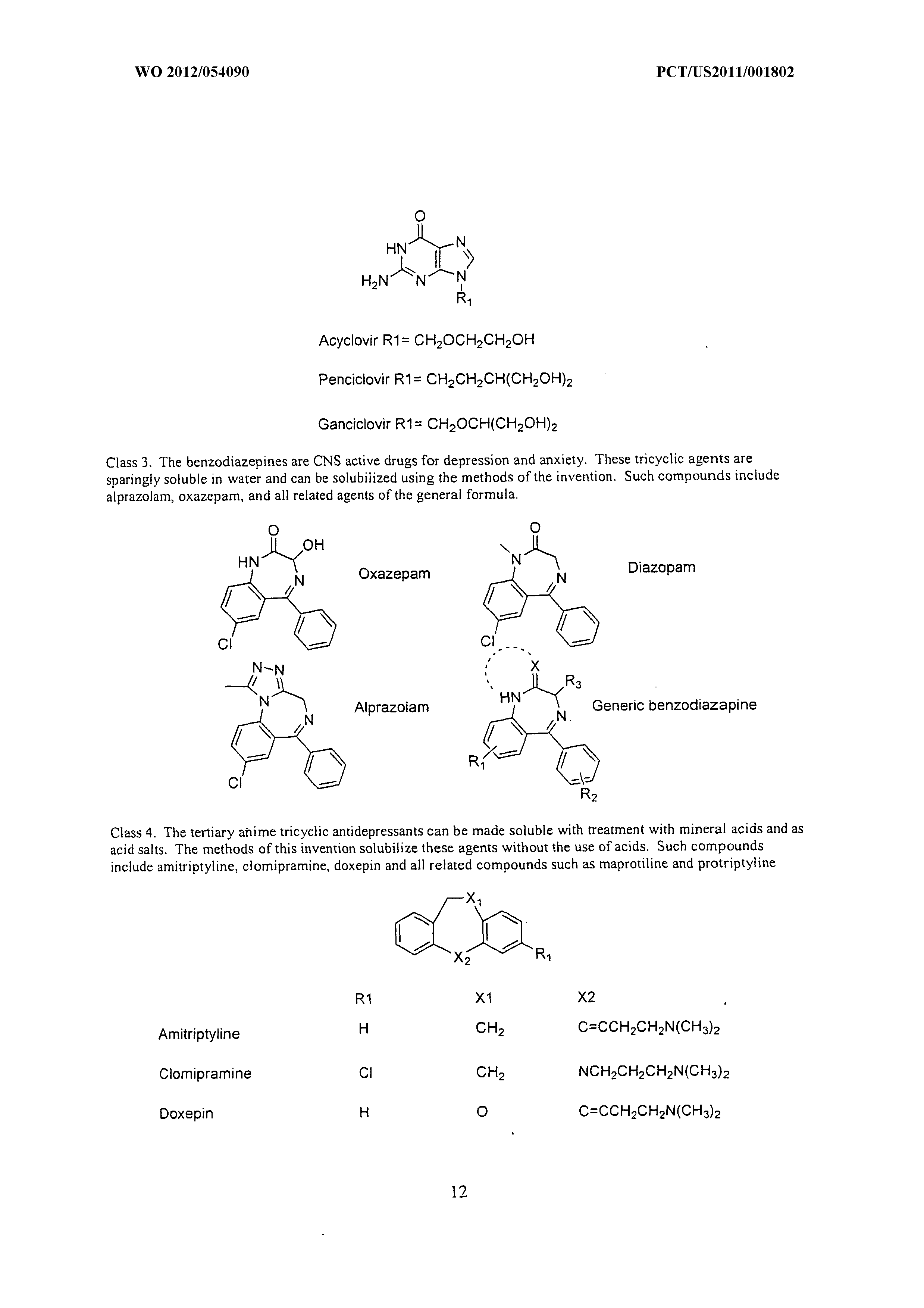
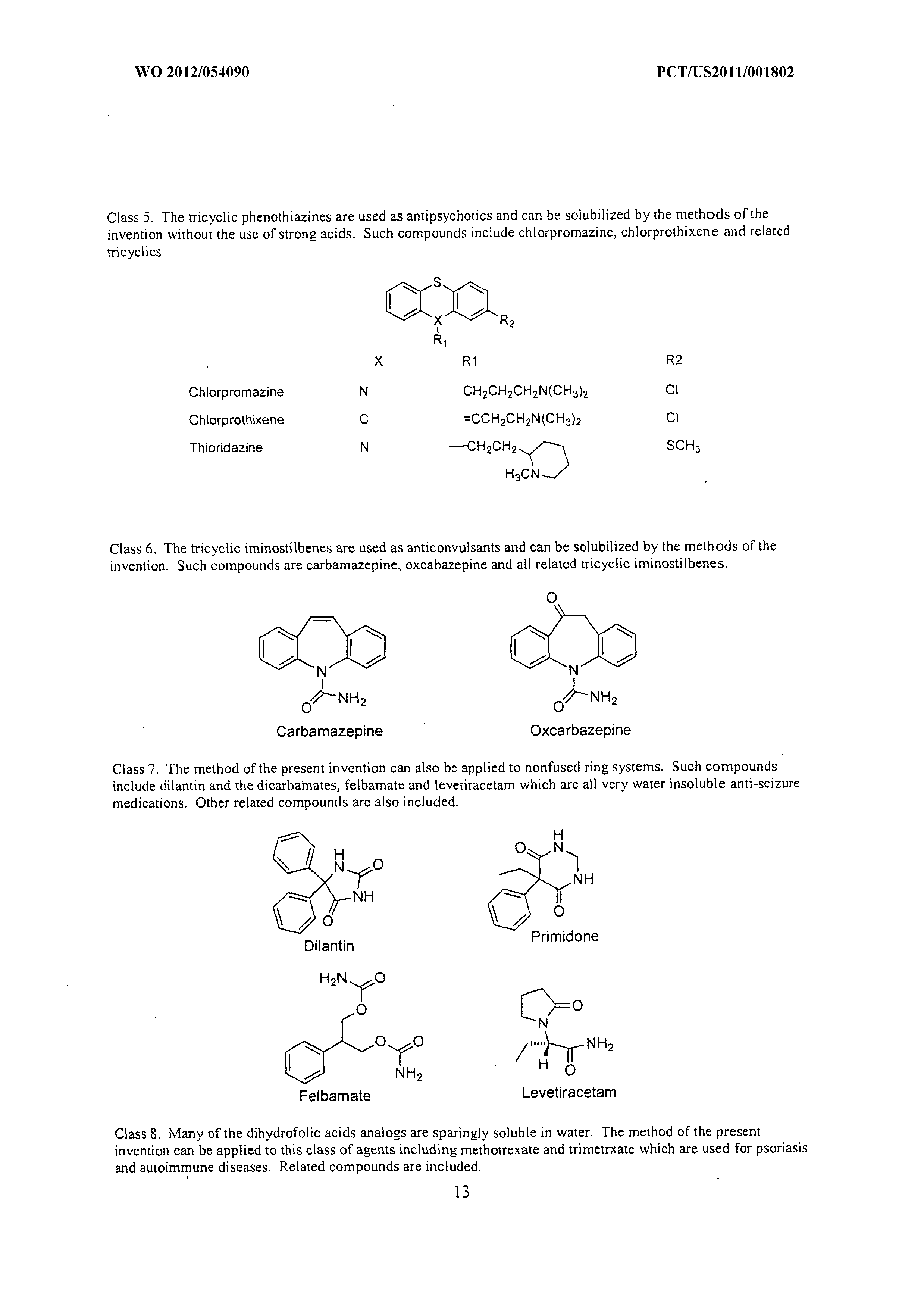
Wo 2012 054090 A1 Methods Of Increasing Solubility Of Poorly Soluble Compounds And Methods Of Making And Using Formulations Of Such Compounds The Lens Free Open Patent And Scholarly Search
www.lens.org