If The Formula Mass Of A Compound Is 11279 Amu Which Of These Could It Be
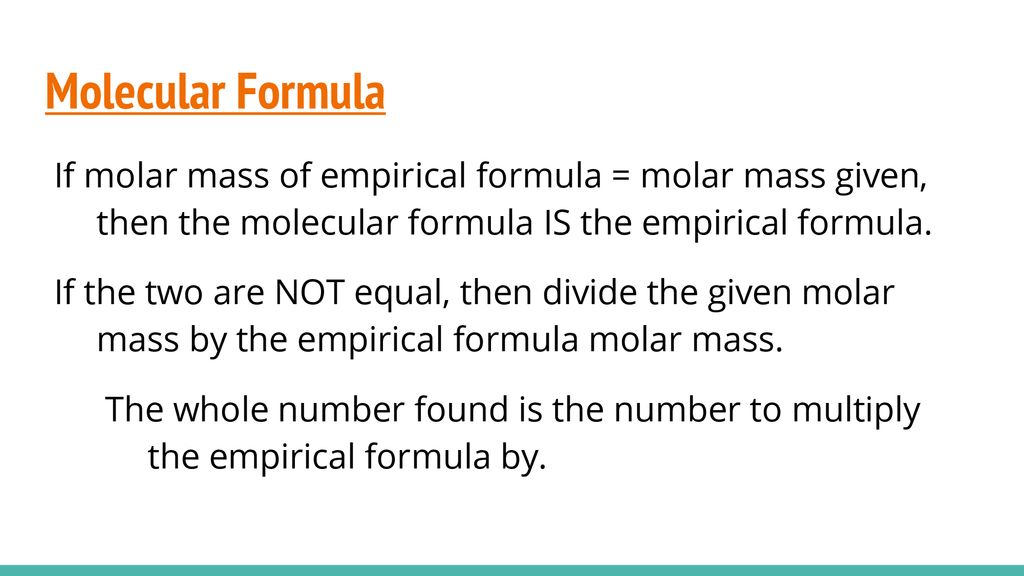
The subscripts in the actual molecular formula are 242.
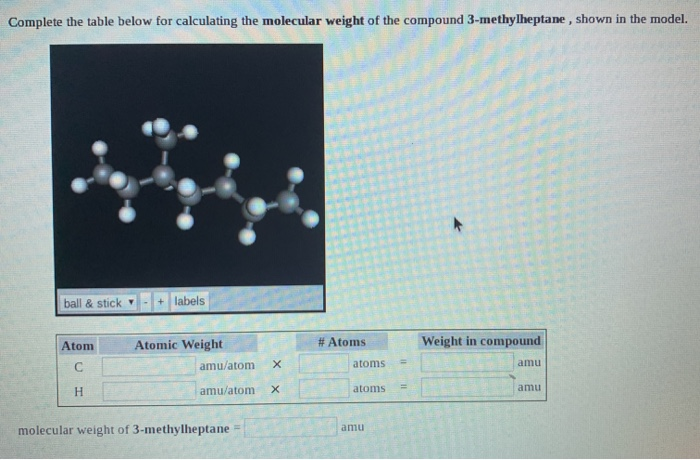
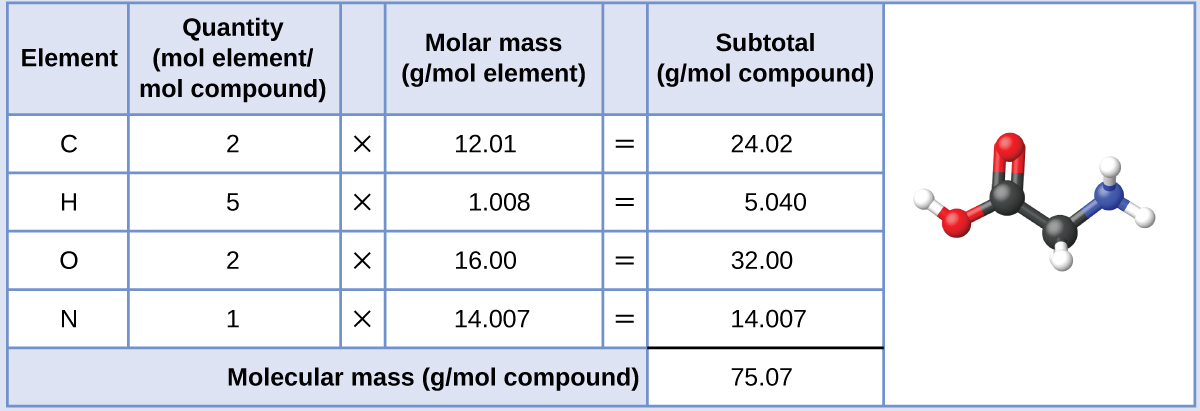
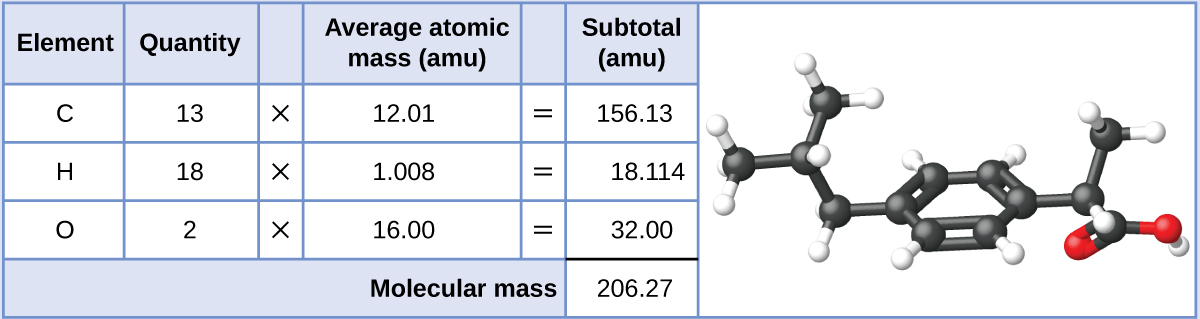
If the formula mass of a compound is 11279 amu which of these could it be. Computing molecular mass for a covalent compound ibuprofen c 13 h 18 o 2 is a covalent compound and the active ingredient in several popular nonprescription pain medications such as advil and motrinwhat is the molecular mass amu for this compound. What is the formula mass of the compound in amu. The molar mass is the same numeric value as the formula mass but it is expressed using the unit gmol.
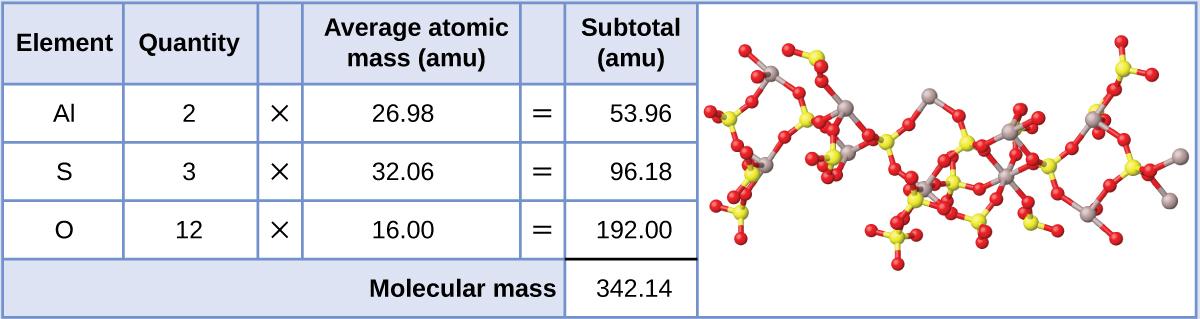
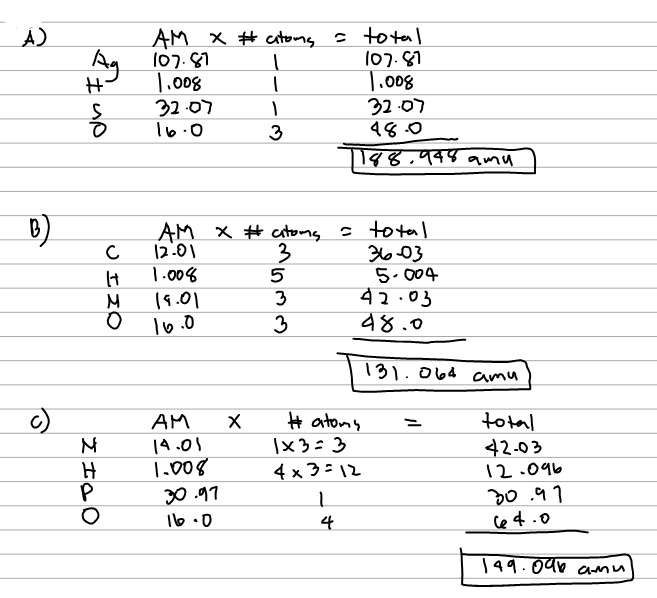
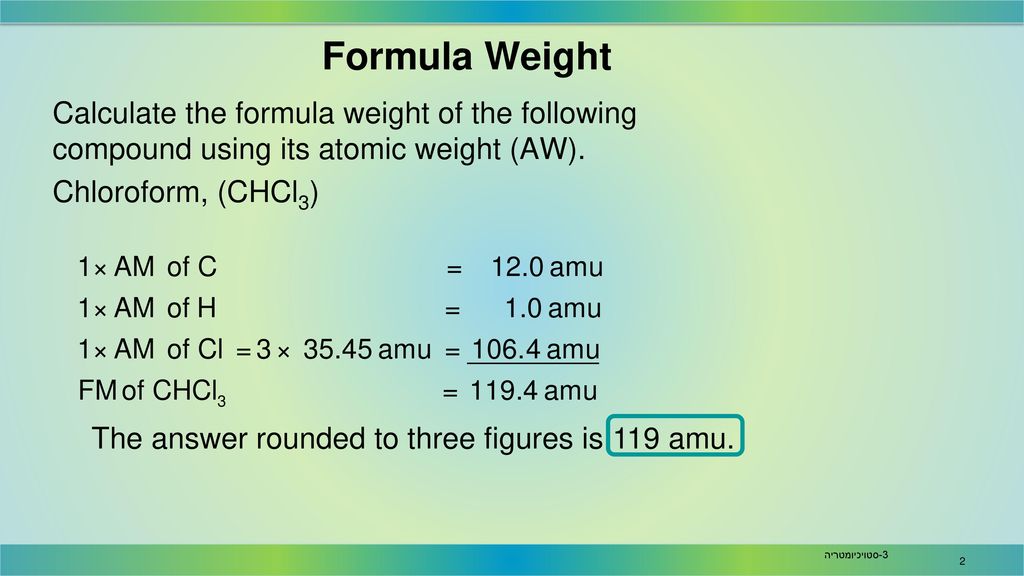
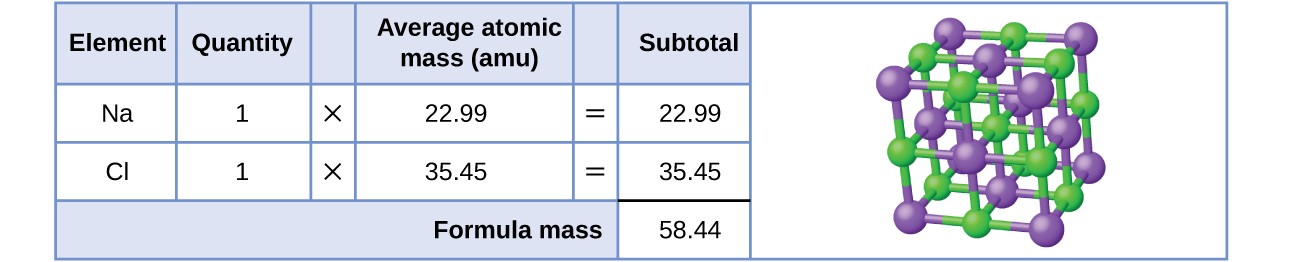
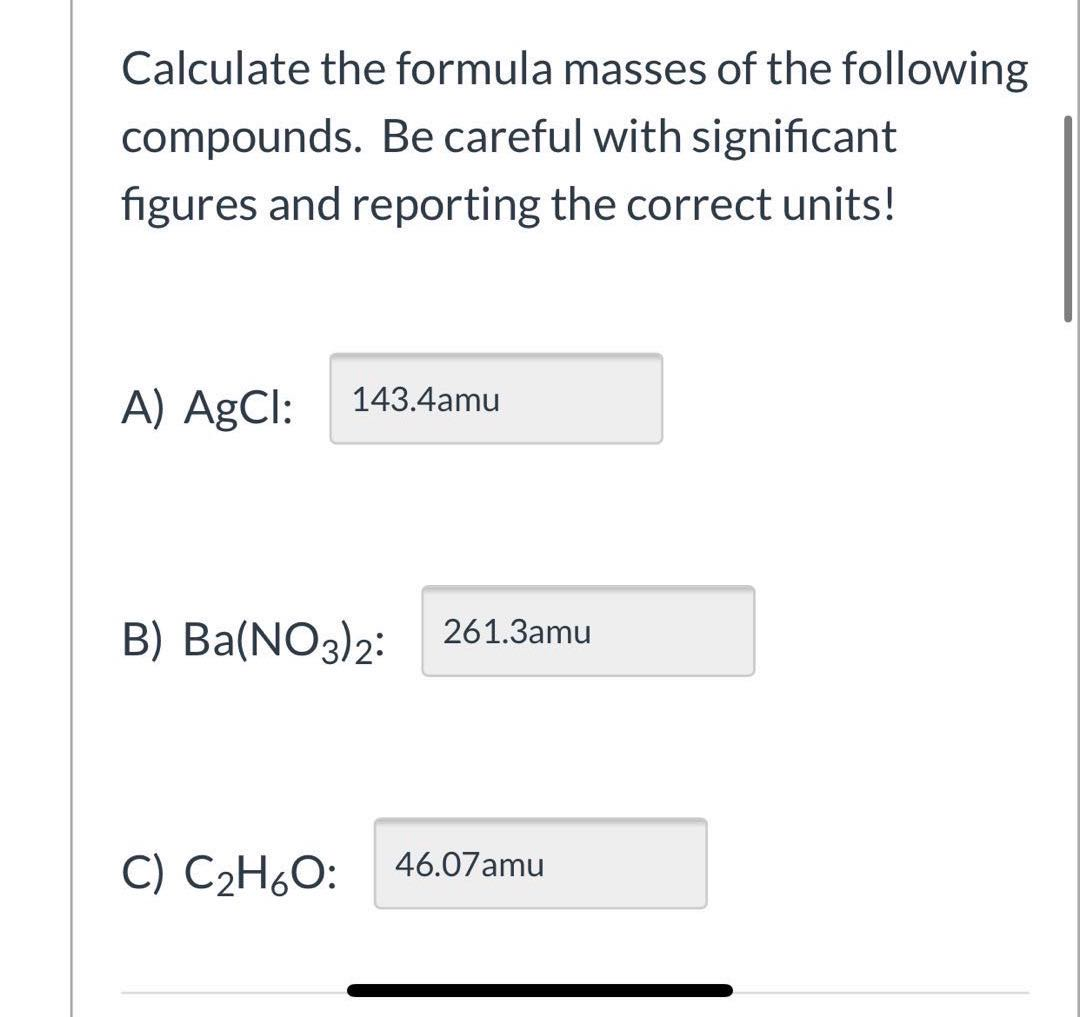
Here we use the masses to two decimal places. The atomic mass of h is therefore is equal to amu whereas the atomic mass of c is equal to amu. To two decimal places the formula mass of nacl.
Let us start by calculating the formula mass of sodium chloride nacl. Relative formula mass definition. Following naming rules question 10 of 25 if the formula mass of a compound is 11279 amu which of these could it be.
In hydrogen peroxide there is 1 part hydrogen for every 16 parts oxygen. Placed the 1st step of the. This simply means the calculation is performed using relative atomic weight values for the elements which are based on the natural isotopic ratio of elements found in earths atmosphere and crust.
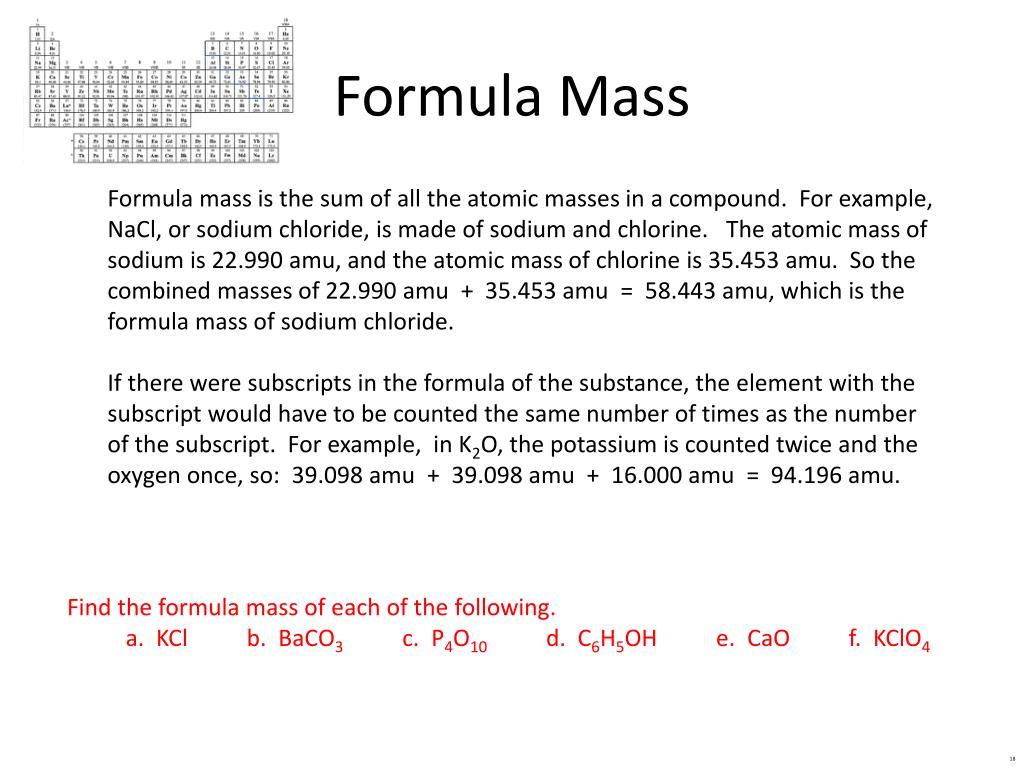
A related term you should know is relative formula mass relative formula weight. Aphosphorus dioxide bsodium nitrate chydrochloric acid dammonium phosphate enone of the above answer key. Because the mass of hydrogen is 1008 amu and the mass of oxygen is 1600 amu there is one h atom for every o atom.
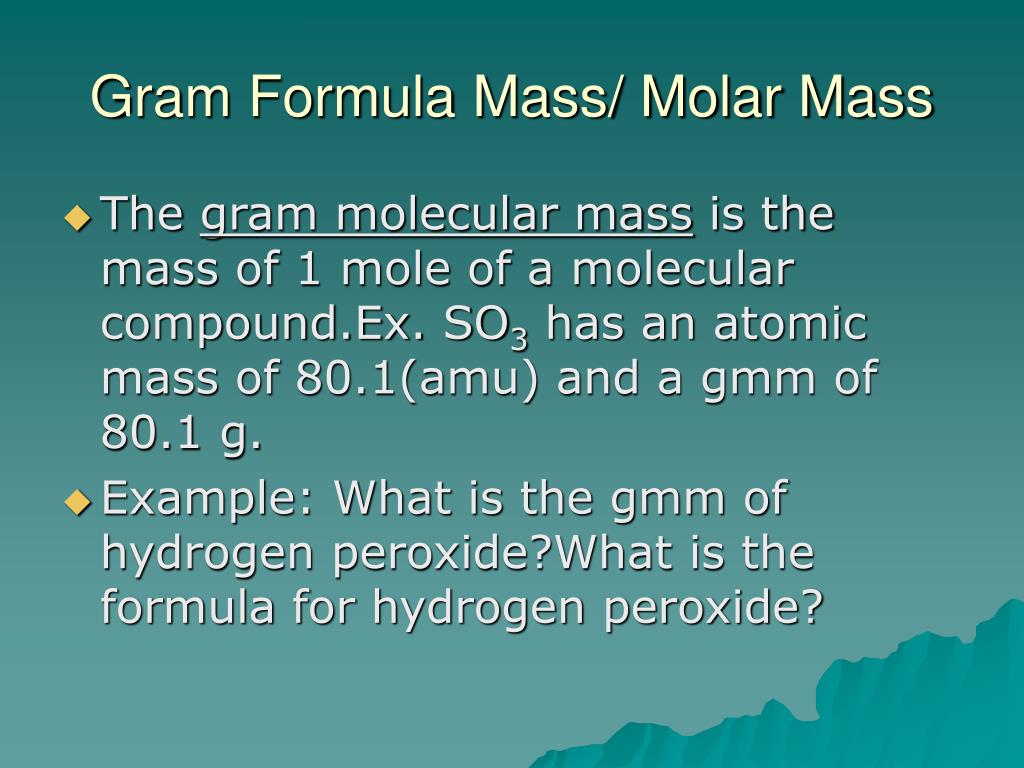
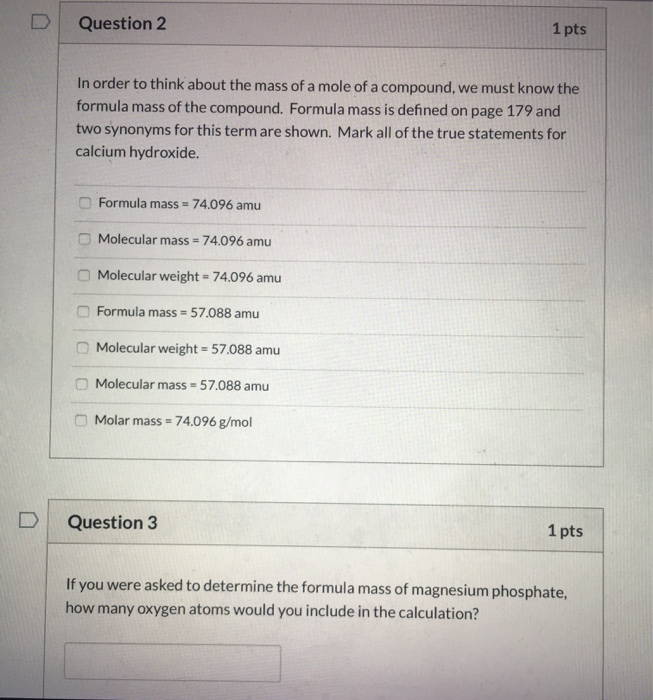
The term formula mass and the term molar mass mean slightly different ideas but only on a technical basis. C9 d3 e1 answer key. The molecular formula shows the actual number of atoms in each element.
C question 7 of 25 40 points if the formula mass of a compound is 11279 amu which of these could it be. Molecules of this compound are comprised of 13 carbon atoms 18 hydrogen atoms and 2 oxygen atoms. The formula mass of 78110 amu is the mass of one molecule of the compound expressed in units of amu.
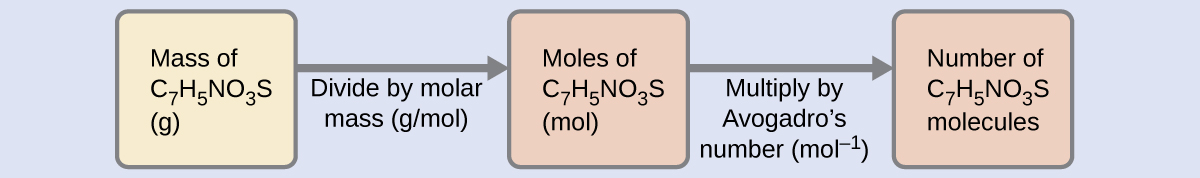
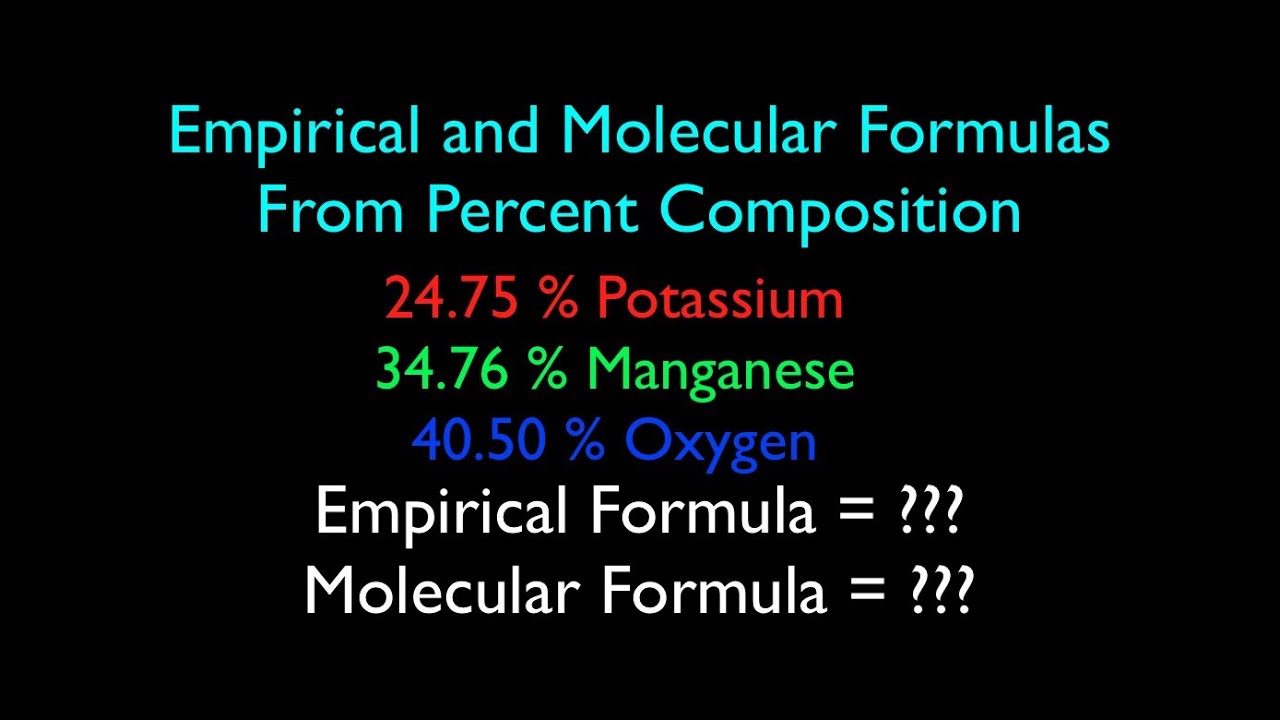
Use the periodic table given and your textbook to answer the question. The molecular formula mass of this compound is 600 amu. Arrange the necessary steps in order for finding the molecular formula of an unknown compound from mass percent data.
Use naming rules and add atom masses question 8 of 25 40 points the ratio of the masses of carbon to oxygen atoms in carbon dioxide is. From that we get the empirical formula ho.
A Molecule With Molecular Weight Of 180 18 G Mol Is Analyzed And Found To Contain 40 00 Carbon 6 72 Hydrogen And 53 28 Oxygen What Are The Empirical And Molecular Formulas Of The Molecule Socratic
socratic.org

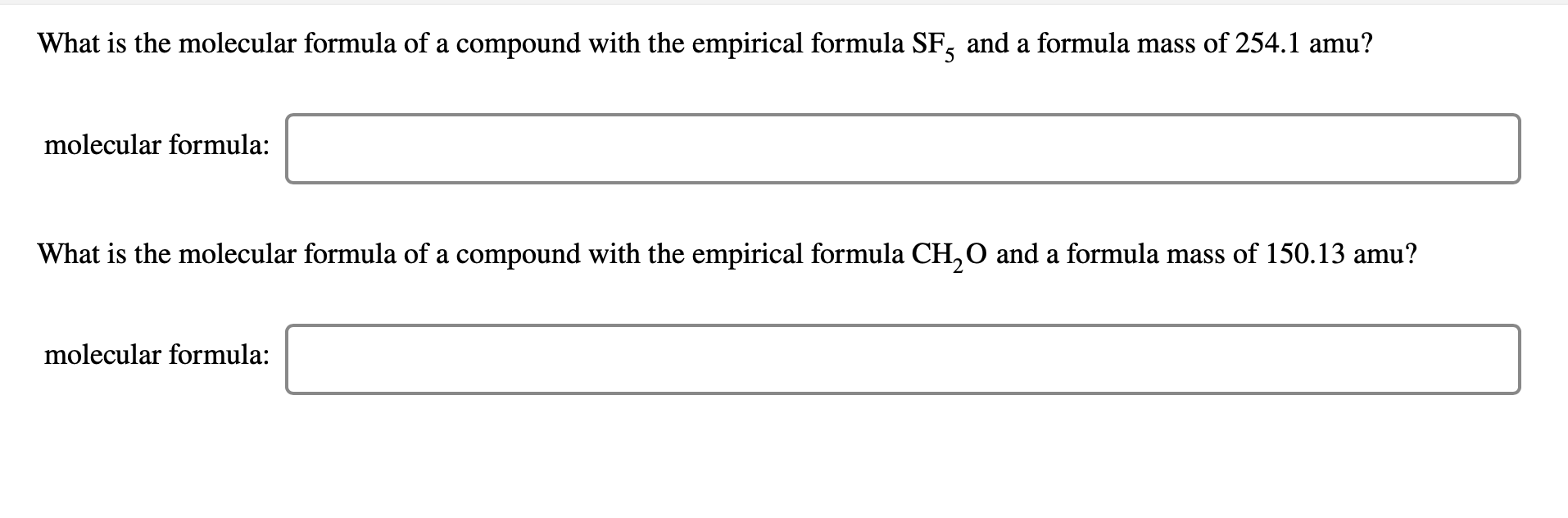
The Empirical Formula Of A Compound Is Ch2o If The Molecular Weight Of The Compound Is 180 The Molecular Formula Is
www.toppr.com

Synthesis Structures And Various Biological Applications Of New Zn Ii Complexes Having Different Coordination Modes Controlled By The Drug Furosemide In Presence Of Bioactive Nitrogen Based Ligands Raymoni 2019 Applied
onlinelibrary.wiley.com






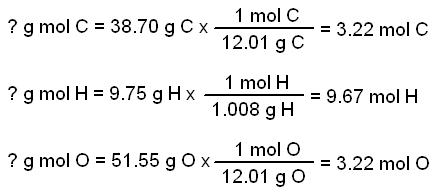









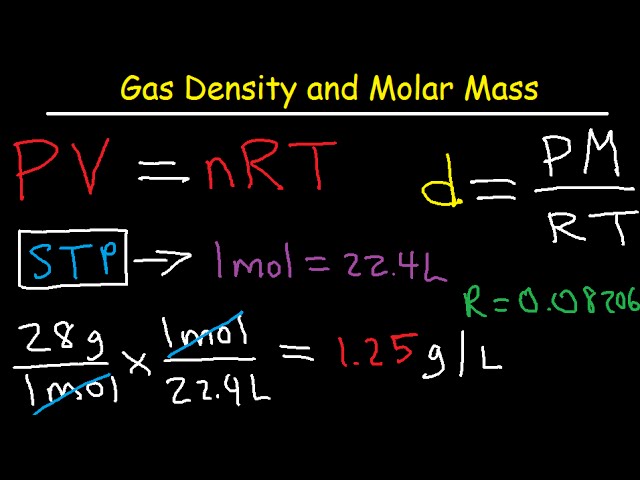














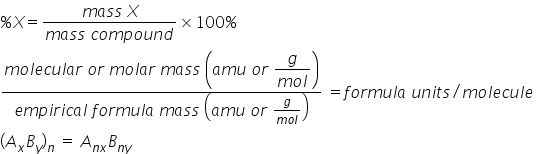










:max_bytes(150000):strip_icc()/GettyImages-175532236-c614b233b7e84d5487cad8b280f365a4.jpg)

















:max_bytes(150000):strip_icc()/mass-percent-composition-example-609567_V2-01-89c18a9d30ea43b494d09b81f7ffefc1.png)