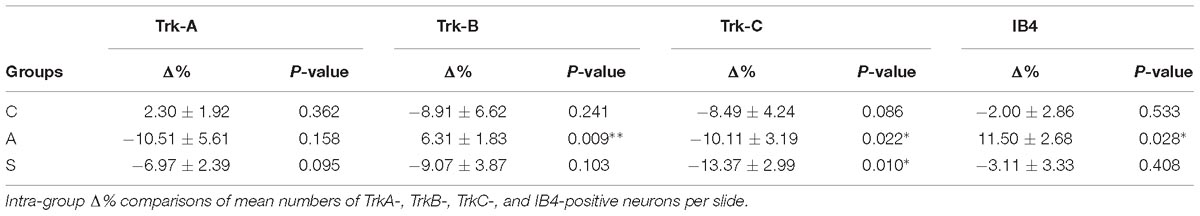
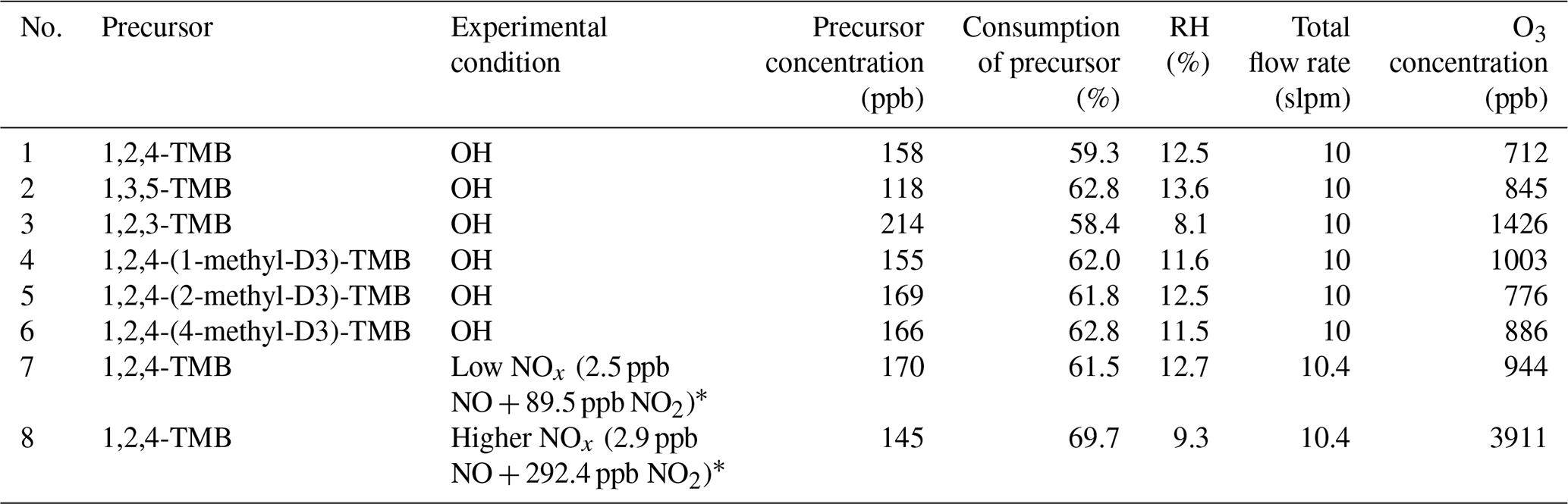
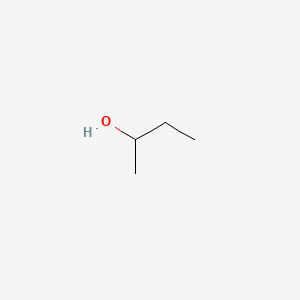
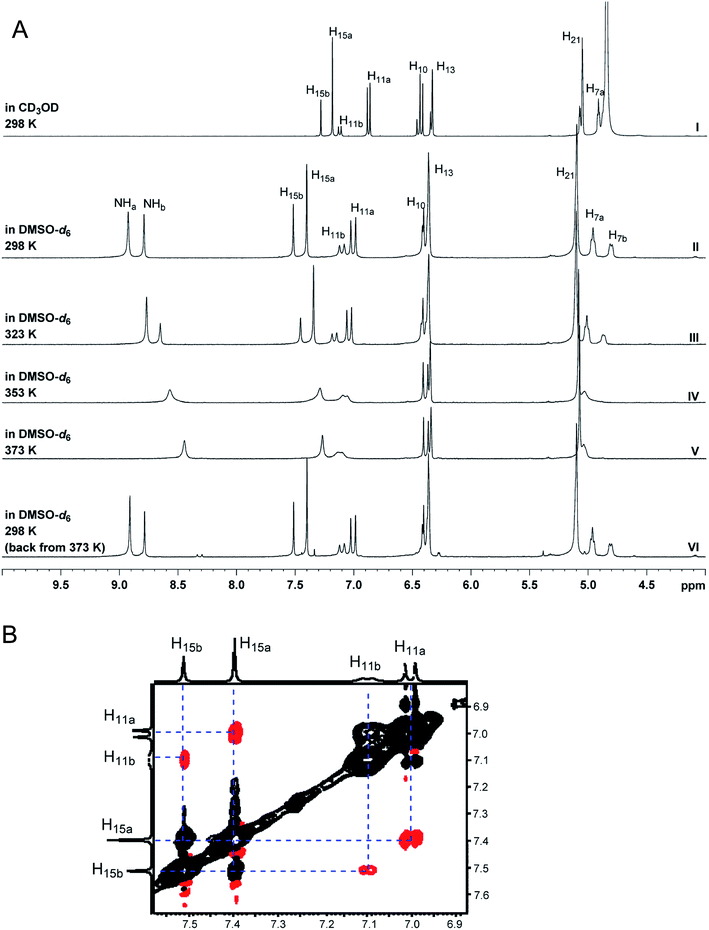
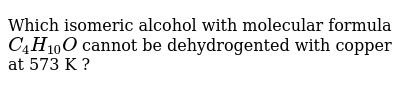Deduce The Molecular Formula Of The Alcohol Whose Mr158
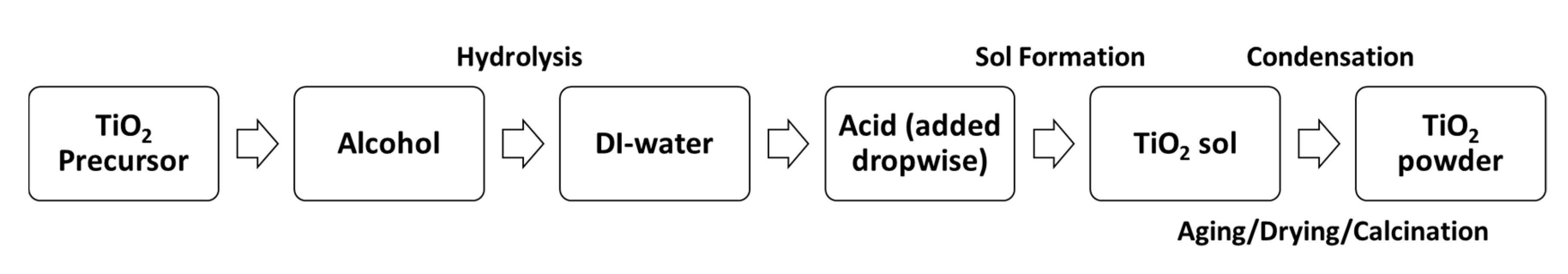
Catalysts Free Full Text Synthesis And Surface Modification Of Tio2 Based Photocatalysts For The Conversion Of Co2 Html
www.mdpi.com
Order the elements according to the general rules for naming ionic and molecular compounds.

Deduce the molecular formula of the alcohol whose mr158. The molecular formula is the representation of the actual whole number ratio between the elements of the compound. Using the molecular ion to find the relative formula mass. A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule using chemical element symbols numbers and sometimes also other symbols such as parentheses dashes brackets commas and plus and minus signs.
A few compounds have mass spectra which dont contain a molecular ion peak because all the molecular ions break into fragments. Nutrasweet is 5714 c 616 h 952 n and 2718 o. Deduce the name and the structural formula of the or.
Write the empirical formula by attaching these whole number mole ratios as subscripts to the chemical symbol of each element. The molecule may have a molecular formula of ch 2 o c 2 h 4 o 2 c 3 h 6 o 3 or the like. For example a molecule with the empirical formula ch 2 o has an empirical formula mass of about 30 gmol 12 for the carbon 2 for the two hydrogens 16 for the oxygen.
This step by step tutorial shows how to calculate the empirical and molecular formulas for a compound. Deduce the molecular formula of the alcohol whose mr 158. If percentages are given assume that the total mass is 100 grams so that.
Deduce the formula of the compound which has the composition 0013 moles of iodine atoms and 0065 moles of uorine atoms. These are limited to a single typographic line of symbols which may include. Iii deduce the molecular formula of the alcohol whose m r 158.
When a substituent such as a halogen or hydroxy group bonds to an alkane molecule one of the carbonhydrogen bonds of the molecule is converted to a carbonsubstituent bond. The molar mass of nutrasweet is 29430 gmol start with the number of grams of each element given in the problem. As a result the compound may have a gram molecular mass of 30 gmol 60 gmol.
Thus octane an eightcarbon alkane has a molecular formula of c 8 h 18 and a structural formula of. For example when methane reacts with chlorine. What is the empirical formula of a substance that is 400 carbon 67 hydrogen and 533 oxygen by mass.
Show your workinghow to find relative atomic mass of hydrogen. The empirical formula of a chemical compound is a representation of the simplest whole number ratio between the elements comprising the compound.

Three Component Reaction An Efficient Synthesis And Reactions Of 3 4 Dihydropyrimidin 2 1h Ones And Thiones Using New Natural Catalyst Abstract Europe Pmc
europepmc.org

Phosphorus Containing Carbons Preparation Properties And Utilization Sciencedirect
www.sciencedirect.com

Pdf Dissymmetry Factor Spectral Analysis Can Provide Useful Diastereomer Discrimination Chiral Molecular Structure Of An Analogue Of Crispine A
www.researchgate.net

Mass Spectrometry Introduction Mass Spectra Spectrum Time Of Flight Mass Spectrometer Abundance Of Isotopes Relative Atomic Mass Determination Strontium Chlorine Bromine A Level Chemistry Revision Notes
www.docbrown.info

Nitrogen Containing Constituents Of Black Cohosh Chemistry Structure Elucidation And Biological Activities Abstract Europe Pmc
europepmc.org

Ep0329303b1 Device And Method For Separating Leukocytes From Platelet Concentrate Google Patents
patents.google.com
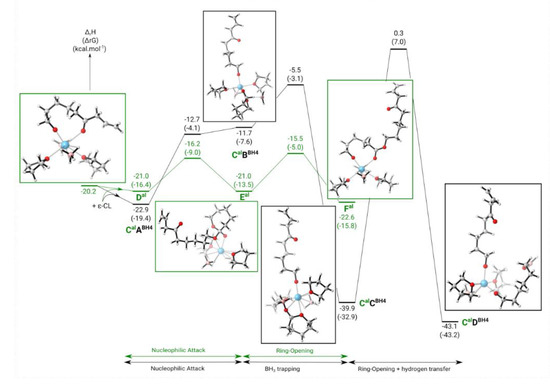
Catalysts Free Full Text Rationalizing The Reactivity Of Mixed Allyl Rare Earth Borohydride Complexes With Dft Studies Html
www.mdpi.com

Pdf Interactions Of Ammonium Smectite With Volatile Organic Compounds From Leachates
www.researchgate.net
Nitrogen Heterocycles Form Peptide Nucleic Acid Precursors In Complex Prebiotic Mixtures Scientific Reports
www.nature.com
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcsxptr Sahq633bw0mf3lxqhqutmsai3s1tldlvnomvv9nsuczc Usqp Cau
encrypted-tbn0.gstatic.com