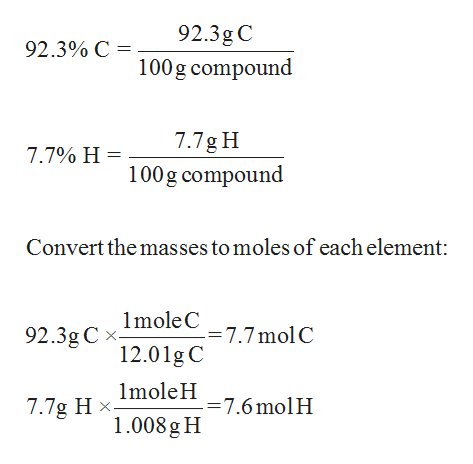C Formula Mass

Empirical And Molecular Formula Questions You Should Be Able To Do These Questions Without A Calculator
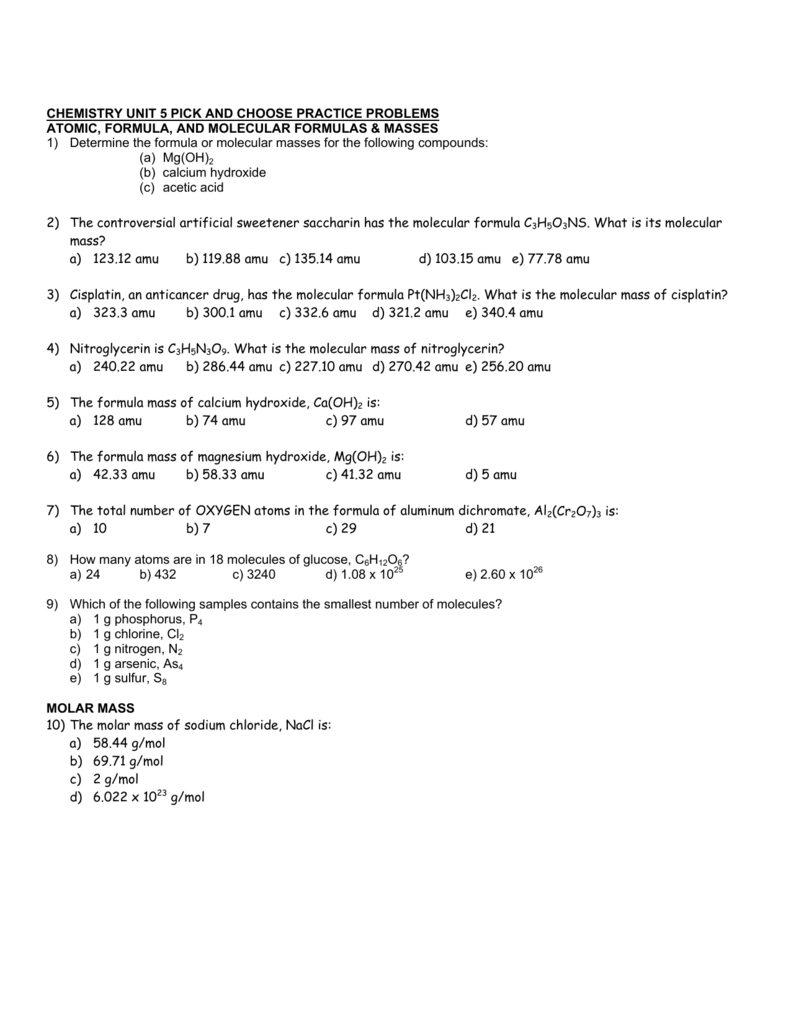
www.scribd.com
Formula in hill system is c.
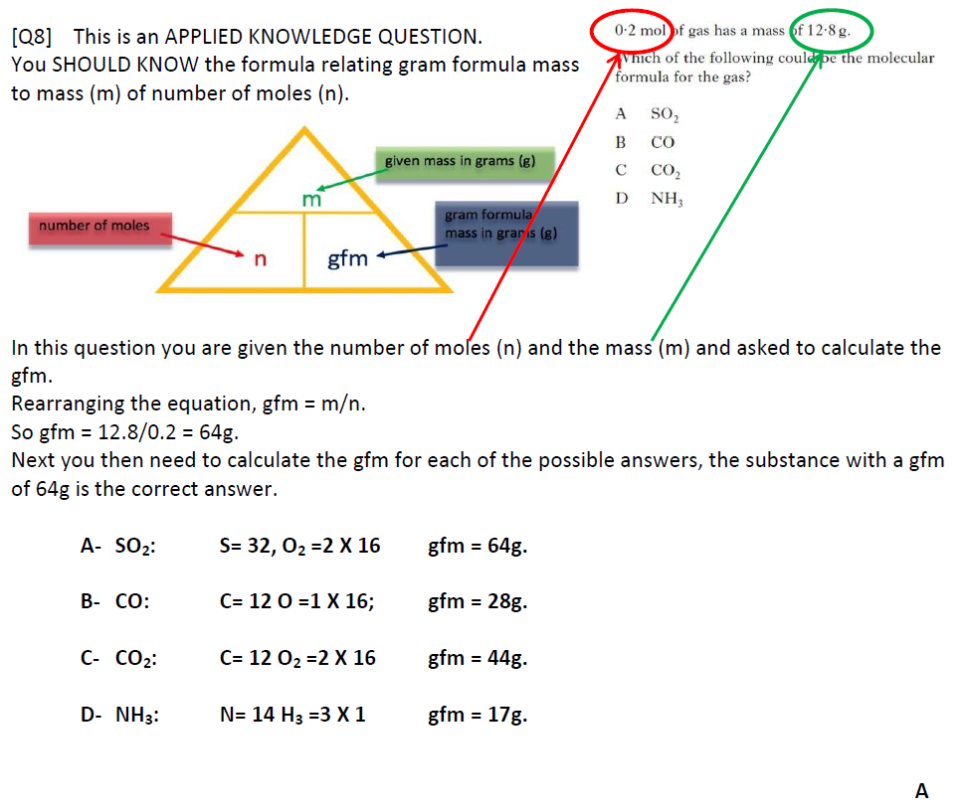
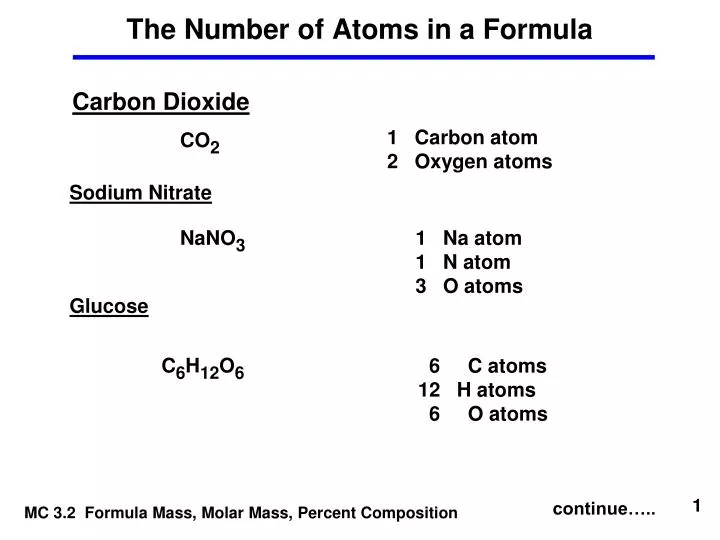
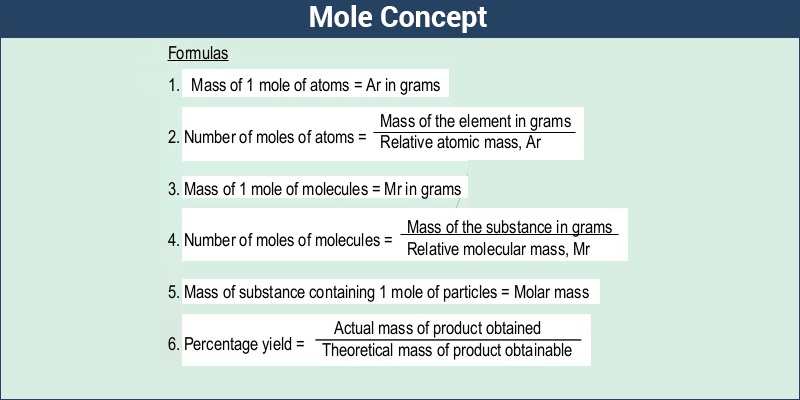
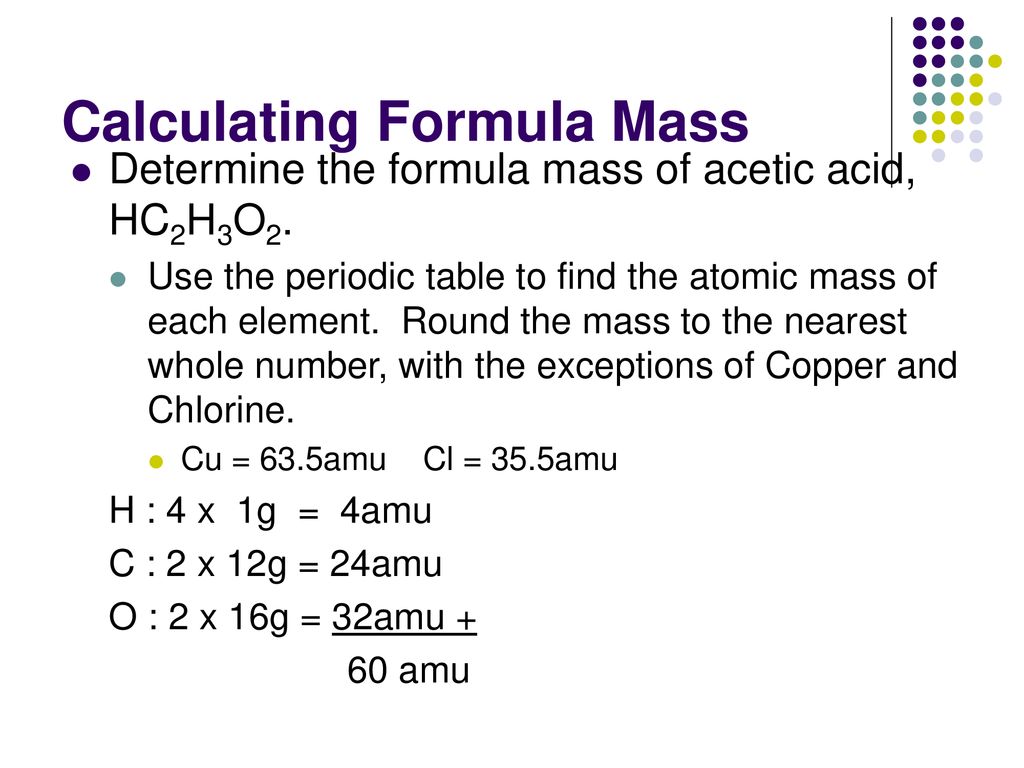
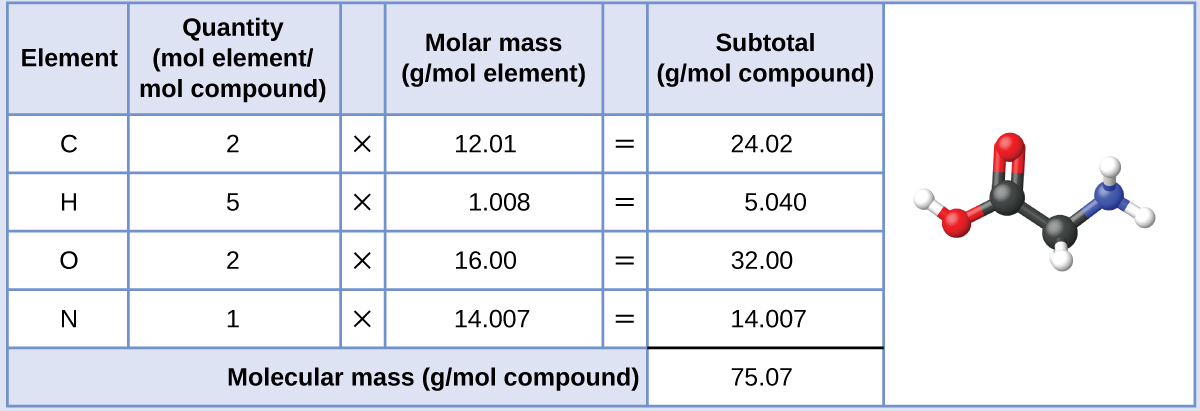
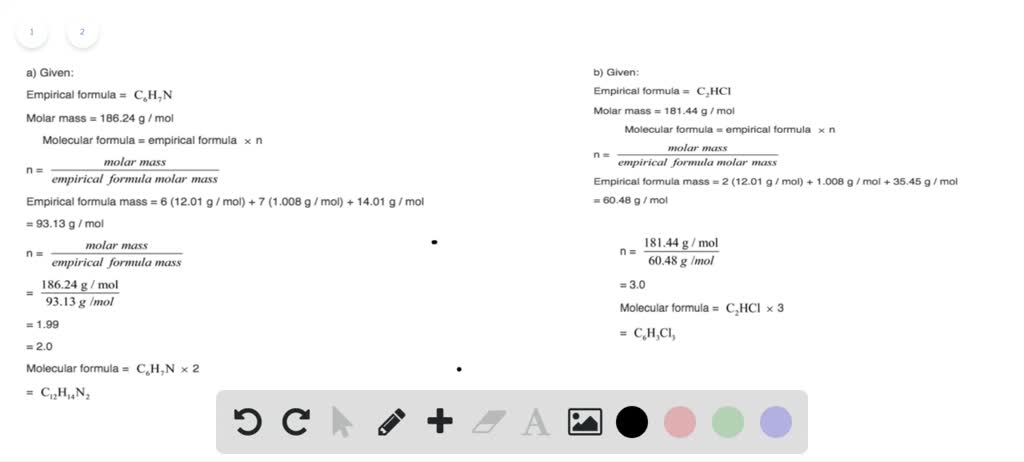
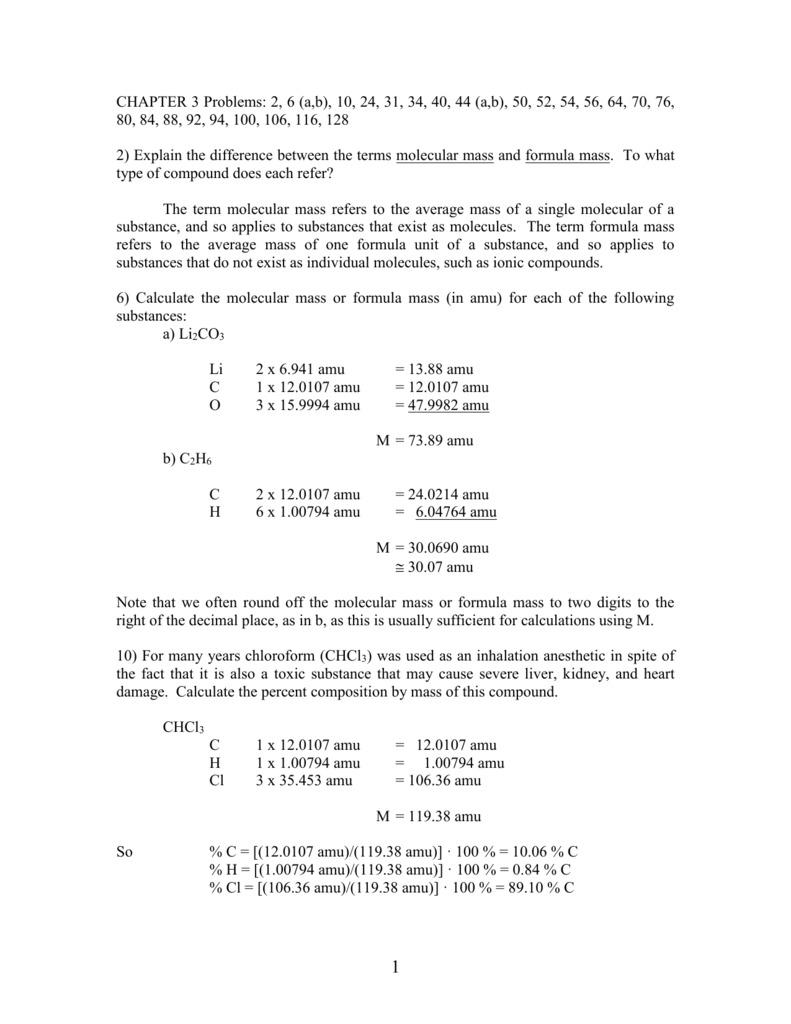
C formula mass. Ca fe mg mn s o h c n na k cl al. The empirical formula for c 4 h 10 is c 2 h 5. The mass of c atom 120107amu the mass of an h atom 10079 amu therefore the formula mass 2 x 120107amu 5 x 10079 amu.
In chemical formula you may use. If the formula used in calculating molar mass is the molecular formula the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the.
Since c3000000 ms c 2 is an extremely large factor. Capitalize the first letter in chemical symbol and use lower case for the remaining letters. 12 16 28.
Emc 2 tells us that the total energy of a non moving body is directly proportional to its mass by a factor of c 2. The formula mass of c 4 h 10. The relation between potential energy mass the acceleration due to gravity and height is given by.
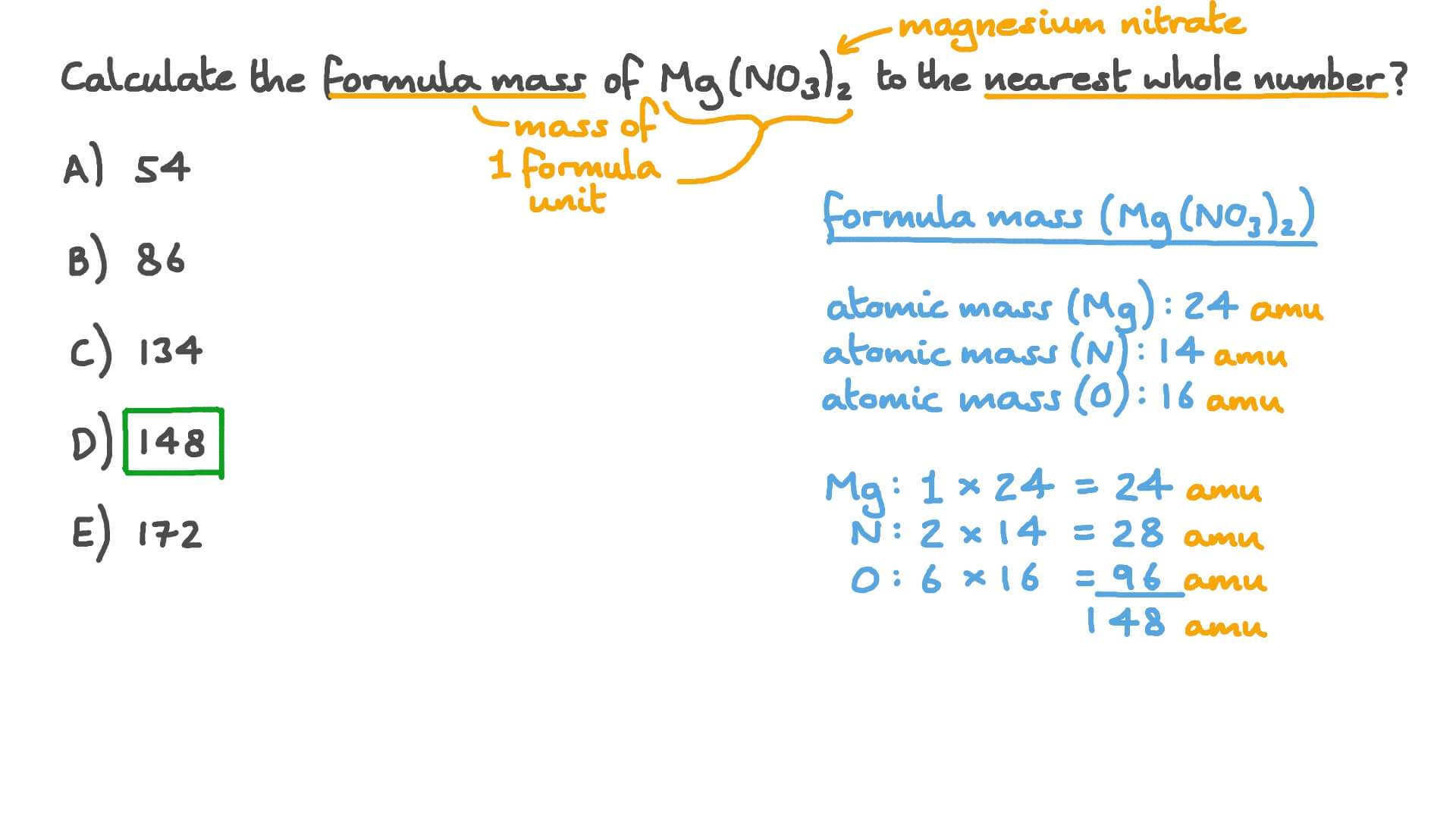
The relative atomic mass of carbon is 12 and of oxygen is 16 so the relative formula mass is. Therefore the formula mass can be calculated as follows. P mgh or m pgh.
Where e energy m mass c speed of light. To find the relative formula mass of sodium oxide na2o you multiply the relative atomic mass of sodium times its subscript and add the value to the relative atomic mass of oxygen. The well known einsteins mass energy relation that provides mass energy formula as.
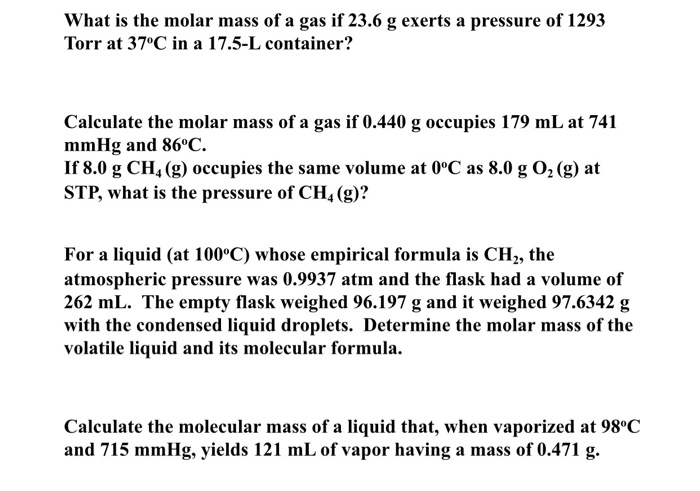
23 x 2 16 62. Computing molar mass molar weight to calculate molar mass of a chemical compound enter its formula and click compute.

Cambridge International As And A Level Chemistry Coursebook With Cd Rom By Cambridge University Press Education Issuu
issuu.com

The Relative Formula Mass Of A Hydrocarbon Is 58 Draw And Name Two Possible Structures Of The Hydrocarbon C 12 0 H 1 0
www.kenyaplex.com

Physical Chemistry General 12 An Organic Compound Containing C H O Has Molar Mass 118 G Mol The Molecular Formula Of The Com Kunduz
kunduz.com